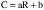-
Start Preamble
Start Printed Page 78746
AGENCY:
Environmental Protection Agency (EPA).
ACTION:
Final rule.
SUMMARY:
This rule establishes 17 new Toxic Substances Control Act (TSCA) health effects test guidelines in the Code of Federal Regulations (CFR). Establishment of these guidelines provides a series of standardized test procedures and is necessary to ensure enforceable test standards in test rules promulgated under section 4 of TSCA. Codification of this series of TSCA test guidelines does not by itself impose obligations upon any person. Obligations are only imposed when these guidelines are cross-referenced in a test rule promulgated under section 4 of TSCA. The TSCA test guidelines are based on the harmonized test guidelines in the unified library for test guidelines issued by the Office of Prevention, Pesticides and Toxic Substances (OPPTS) for use in testing chemical substances to develop data for submission to EPA under TSCA, the Federal Food, Drug, and Cosmetic Act (FFDCA), and the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). The process for developing and amending the harmonized test guidelines includes broad public participation and extensive involvement of the scientific community.
DATES:
This rule is effective on December 15, 2000.
Start Further InfoFOR FURTHER INFORMATION CONTACT:
For general information contact: Barbara Cunningham, Director, Office of Program Management and Evaluation, Office of Pollution Prevention and Toxics (7401), Environmental Protection Agency, 1200 Pennsylvania Ave., NW., Washington, DC 20460; telephone number: (202) 554-1404; e-mail address: TSCA-Hotline@epa.gov.
For technical information regarding this action or related activities contact: Chemical Information and Testing Branch, Chemical Control Division, Office of Pollution Prevention and Toxics (7405), Environmental Protection Agency, 1200 Pennsylvania Ave., NW., Washington, DC 20460; telephone number: (202) 260-8130; e-mail address: ccd.citb@epa.gov.
End Further Info End Preamble Start Supplemental InformationSUPPLEMENTARY INFORMATION:
This final rule establishes 17 new TSCA test guidelines in the series of TSCA test guidelines established in 40 CFR part 799.
I. General Information
A. Does this Action Apply to Me?
You may be particularly interested in this action if you manufacture (defined by statute to include import) or process a chemical substance that could become the subject of a proposed test rule under TSCA section 4. This action does not, however, impose any obligations on anyone until the test guidelines are incorporated in a future test rule that would be proposed under TSCA section 4. Therefore, entities potentially affected by this action may include, but are not limited to:
Type of Entity NAICS Examples of Potentially Affected Entities Chemical Manufacturers or Importers 325, 32411 Persons who manufacture (defined by statute to include import) one or more of the subject chemical substances. Chemical Processors 325, 32411 Persons who process one or more of the subject chemical substances. This listing is not intended to be exhaustive, but rather provides a guide for readers regarding entities likely to be affected by this action. The North American Industrial Classification System (NAICS) codes have been provided to assist you and others in determining whether or not this action might apply to certain entities. If you have any questions regarding the applicability of this action to a particular entity, consult the technical information contact listed under FOR FURTHER INFORMATION CONTACT.
B. How Can I Get Additional Information, Including Copies of this Document and Other Related Documents?
1. Electronically. You may obtain electronic copies of this document, and certain other related documents that might be available electronically, from the EPA Internet Home Page at http://www.epa.gov/. To access this document, on the Home Page select “Laws and Regulations” and then look up the entry for this document under the “Federal Register—Environmental Documents.” You can also go directly to the Federal Register listings at http://www.epa.gov/fedrgstr/.
2. In person. The Agency has established an official record for this action under docket control number OPPTS-42211. The official record consists of the documents specifically referenced in this action, any public comments received during an applicable comment period, and other information related to this action, including any information claimed as confidential business information (CBI). This official record includes the documents that are physically located in the docket, as well as the documents that are referenced in those documents. The public version of the official record does not include any information claimed as CBI. The public version of the official record, which includes printed, paper versions of any electronic comments submitted during an applicable comment period, is available for inspection in the TSCA Nonconfidential Information Center, North East Mall Rm. B-607, Waterside Mall, 401 M St., SW., Washington, DC. The Center is open from noon to 4 p.m., Monday through Friday, excluding legal holidays. The telephone number for the Center is (202) 260-7099.
II. Background
A. What are Test Guidelines?
Test guidelines are a standardized set of test procedures or protocols organized by health effect or other testing endpoint. These guidelines present generally formulated procedures for laboratory testing of an effect or characteristic deemed important for the evaluation of health and environmental hazards of a chemical. These guidelines are designed to, when followed, produce data which are accurate, reliable, and reproducible. Such data are necessary for the regulatory programs under TSCA.
In adding these 17 TSCA test guidelines to the existing series of 11 TSCA test guidelines, EPA recognizes concerns have been expressed about animal testing. EPA is committed to avoiding unnecessary or duplicative animal testing. As part of this commitment, the Agency plays an Start Printed Page 78747important role in the Federal Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) (http://iccvam.niehs.nih.gov/home.htm) whose goals are: (1) To encourage the reduction of the number of animals used in testing; (2) to seek opportunities to replace test methods requiring animals with alternative test methods when acceptable alternative methods are available; and (3) to refine existing test methods to optimize animal use when there is no substitute for animal testing. Further, where testing is needed to develop scientifically adequate data, the Agency is committed to reducing the number of animals used for testing, including, whenever possible, by incorporating in vitro (non-animal) test methods or other alternative approaches that have been scientifically validated and have received regulatory acceptance. EPA considers these goals and commitments to be important considerations in developing health effects data; however, they must be balanced with the essential need to conduct scientifically sound chemical hazard/risk assessments in support of the Agency's mission to protect human health and the environment.
The completion of this series of 28 TSCA test guidelines in part 799 provides EPA with a range of guidelines available for cross-referencing in TSCA actions. Several of these guidelines include in their design elimination of animals or reduction in the number of animals needed to conduct the tests. Some of the methods are designed to only develop data on chemical/physical properties. In addition, one of the guidelines involves the development of metabolism and pharmacokinetics data which could facilitate route-to-route extrapolations to existing (e.g., oral route) data and thus involve fewer test animals as compared to developing new data by, for example, the inhalation route. EPA believes that using these test guidelines will result in use of fewer test animals when it becomes necessary to conduct testing to fill identified data needs and will yield scientifically sound data.
B. What are TSCA Test Guidelines?
TSCA test guidelines are guidelines which were established to meet the regulatory needs of TSCA, particularly the needs of the TSCA section 4 testing program. The TSCA section 4 testing program is a regulatory program which is based on the promulgation of rules requiring certain persons identified in the rule, usually manufacturers and processors of the chemical to conduct testing of the chemical specified in the rule. Section 4(b)(1)(B) of TSCA specifically requires that test rules promulgated under the section 4 include “standards for the development of test data for such substance or mixture * * *.” These “standards for the development of test data” specify how the study is to be conducted, what data will be collected, and how the data will be analyzed. Each test rule must specify such “test standards” which contain specifications for testing. Section 4(b)(1) of TSCA describes the elements which must be described in these test standards.
The Agency has found that most of these elements can be standardized into the common set of protocols which EPA defines as “test guidelines.” These guidelines are organized by testing endpoint. The test rule itself can add or subtract to the requirements of the test guidelines in order to meet the unique testing circumstances for the particular chemical substance.
C. How are TSCA Test Guidelines Used?
The Agency uses this system of standardized guidelines, organized by testing endpoint and codified in a subpart of this part for use in cross-referencing in a TSCA section 4 action. When a section 4 test rule is promulgated, the test rule cross-references the appropriate TSCA test guideline for the bulk of the testing requirements. In this context, the public is given notice of, and an opportunity to comment on, these guidelines as they are applied in chemical-specific test rules. This approach eliminates the need to repeat the same test specifications for each substance-specific test rule since most of the specifications for testing do not change across substances. The test specifications in a guideline can be varied, when necessary, to the specific requirements of a test rule by language in the test rule itself.
D. Where Did the TSCA Test Guidelines Come From?
The TSCA test guidelines series were first promulgated in 1985 (50 FR 39252, September 27, 1985) and were established in 40 CFR parts 795 through 798. The Agency has over time amended and improved these guidelines (52 FR 19072, May 20, 1987) and in some cases revoked those guidelines which had not been cross-referenced in any test rules (60 FR 31917, June 19, 1995) (FRL-4955-2)).
In 1991, EPA began an effort to blend the testing guidance and requirements that existed in the Office of Pollution Prevention and Toxics (OPPT) appearing in 40 CFR parts 795 through 798, the Office of Pesticides Programs (OPP) guidelines which appeared in publications of the National Technical Information Service (NTIS), and the guidelines published by the international Organization for Economic Cooperation and Development (OECD). The product of this effort would be one set of guidelines which would be thus blended or “harmonized.” These harmonized guidelines would then be made available to the EPA, other government agencies, and the public through the World Wide Web (Internet) and would be accessible by anyone with a personal computer and the ability to connect to the Internet. The EPA Internet web site would be the site and publication source for the “OPPTS Harmonized Guidelines” at http://www.epa.gov/opptsfrs/home/guidelin.htm.
In addition, EPA has published three new OPPTS harmonized test guidelines for three health effects end points. These three guidelines (with their OPPTS harmonized guideline reference) are: (1) Repeated dose 28-day oral toxicity study in rodents (OPPTS 870.3050), (2) Reproduction/developmental toxicity screening test (OPPTS 870.3550), and (3) Combined repeat dose toxicity study with the reproduction/developmental toxicity screening test (OPPTS 870.3650). Their publication was announced in the Federal Register of July 13, 2000 (65 FR 43329) (FRL-6393-5), with the guidelines available from the EPA Internet web site at http://www.epa.gov/opptsfrs/home/guidelin.htm. By adopting these combined testing guidelines, which incorporate more than one endpoint, the Agency is acknowledging the desirability of reducing costs and numbers of animals required to meet the Agency's testing needs. EPA recommends the use of combined protocols wherever feasible to meet data development requirements.
E. How were these OPPTS Harmonized Test Guidelines Developed?
The OPPTS harmonized test guidelines for health effects endpoints were first drafted by EPA scientists for specific testing endpoints. These drafts were reviewed by other EPA experts and, in some instances, presented at domestic and international colloquia in order to solicit the views of recognized experts and the regulated community. These draft harmonized guidelines were made available on the Internet as public drafts and a notice was published in the Federal Register of June 20, 1996 (61 FR 31522) (FRL-5367-7) announcing the availability of these draft guidelines soliciting public comment. Start Printed Page 78748
After review of the public drafts, EPA published the final OPPTS harmonized guidelines for the health effects endpoints on the Internet and announced their availability to the public in the Federal Register of August 5, 1998 (63 FR 41845) (FRL-5740-1). EPA published the rationale for the changes made in finalizing the June 1996 OPPTS “Public Draft” guidelines to the August 1998 OPPTS “Final” guidelines in a document entitled “Overview and Summary of Changes made in the Harmonization of OPPTS 870 Toxicology Guidelines with OECD Guidelines” (which is available at http://www.epa.gov/opptsfrs/home/guidelin.htm).
F. What is Done to Make TSCA Test Guidelines From the OPPTS Harmonized Test Guidelines?
Harmonization has resulted in significantly improved guidelines. However, creating a single set of guidelines which can be used by both OPP, in its administration of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), and the Federal Food, Drug and Cosmetic Act (FFDCA), and the Office of Pollution Prevention and Toxics (OPPT), which administers TSCA presents certain challenges. Under FIFRA, test guidelines are used in an interactive process between the Agency and registrants seeking registration of pesticides or food residue tolerances. Flexibility to tailor required testing to individual circumstances is critical, and the Agency has considerable discretion to determine whether submitted test results are adequate to support the requested action. Under this scheme, registrants have an intrinsic motivation to conduct well-grounded testing. Thus, pesticide testing protocols tend to have few absolute requirements specifying the details of the conduct of the testing.
Under section 4 of TSCA, on the other hand, the Agency imposes prescriptive test requirements using notice and comment rulemaking. Rules promulgated under section 4 of TSCA must specify classes of affected parties and specify the standards to be followed by these parties in conducting the required testing. In contrast to FIFRA, the Agency does not interact with companies on an individual basis in designing the testing requirements.
TSCA section 4 rulemakings typically take years to complete. Without initiating another rulemaking process, the Agency has the ability to require further testing only if the tests were not conducted in accordance with the procedures specified in the test rule. In addition, the Agency has an alternative process of negotiating TSCA testing requirements via enforceable consent agreements (ECAs), but these agreements require the consent of all the parties involved. Under TSCA section 4 enforceable test standards, much in the conduct of these test protocols is left to the judgment of those professionals conducting the testing. EPA believes that certain provisions must be mandatory whenever the guidelines are cross-referenced in specific test rules.
Therefore, the Agency has used the OPPTS harmonized test guidelines developed using the public notice and comment process to create the TSCA-specific test guidelines which are the subject of this rule. TSCA section 4 test rules now cross-reference only the part 799 guidelines rather than the older, non-harmonized guidelines established in 40 CFR parts 795 through 798 mostly in 1985. The only significant difference between the TSCA test guidelines and the OPPTS harmonized test guidelines is that certain discretionary procedures in the OPPTS harmonized test guidelines are made mandatory (i.e., the guideline states that they “must” be carried out) in order to ensure the enforcibility of the test standard.
EPA promulgated the first set of guidelines in the new part 799 guidelines series in a Federal Register document published on August 15, 1997 (62 FR 43820) (FRL-5719-5). Eleven health effects guidelines were published, including those for inhalation toxicity, developmental toxicity, reproductive effects, carcinogenicity, mutagenicity, and immunotoxicity. EPA amended 7 of these 11 guidelines in a Federal Register document published on June 30, 1999 (64 FR 35072) (FRL-6067-4). These amendments reflected changes made to the corresponding OPPTS harmonized guideline.
III. What Action is Being Taken?
EPA is adding 17 new health effects test guidelines to 40 CFR part 799. These 17 new guidelines are listed in the following table 1 with the OPPTS harmonized guideline from which it was developed:
Start Printed Page 78749Table 1
New TSCA test guideline name (and cite) Original OPPTS harmonized guideline name (and cite) TSCA partition coefficient (n-octanol/water) shake flask method (799.6755) Partition coefficient (n-octanol/H2O) shake flask method (830.7550) TSCA partition coefficient (n-octanol/water), generator column method (799.6756) Partition coefficient (n-octanol/H2O), generator column method (830.7560) TSCA water solubility: Column elution method; shake flask method (799.6784) Water solubility: Column elution method; shake flask method (830.7840) TSCA water solubility, generator column method (799.6786) Water solubility, generator column method (830.7860) TSCA acute oral toxicity (799.9110) Acute oral toxicity (870.1100) TSCA acute dermal toxicity (799.9120) Acute dermal toxicity (870.1200) TSCA acute inhalation toxicity (799.9130) Acute inhalation toxicity (870.1300) TSCA repeated dose 28-day oral toxicity study in rodents (799.9305) Repeated dose 28-day oral toxicity study in rodents (870.3050) TSCA 90-day oral toxicity in rodents (799.9310) 90-day oral toxicity in rodents (870.3100) TSCA 90-day dermal toxicity (799.9325) 90-day dermal toxicity (870.3250) TSCA reproduction/developmental toxicity screening test (799.9355) Reproduction/developmental toxicity screening test (870.3550) TSCA combined repeated dose toxicity study with the reproduction/developmental toxicity screening test (799.9365) Combined repeated dose toxicity study with the reproduction/developmental toxicity screening test (870.3650) TSCA chronic toxicity (799.9410) Chronic toxicity (870.4100) TSCA combined chronic toxicity/carcinogenicity (799.9430) Combined chronic toxicity/carcinogenicity (870.4300) TSCA in vitro mammalian chromosome aberration test (799.9537) In vitro mammalian chromosome aberration test (870.5375) TSCA developmental neurotoxicity (799.9630) Developmental neurotoxicity (870.6300) TSCA metabolism and pharmacokinetics (799.9748) Metabolism and pharmacokinetics (870.7485) IV. How are the New TSCA Test Guidelines Different From the OPPTS Harmonized Test Guideline From Which they were Derived?
EPA developed the TSCA test guideline from the original OPPTS test guideline shown in the right column of table 1 in Unit III. In keeping with the policy of using a unified set of test guidelines across OPPTS, only minimal changes were made to the OPPTS harmonized guidelines in the development of the TSCA guidelines. These minimal changes consisted of deleting references to FIFRA in the TSCA guidelines, where it was believed by the Agency to be irrelevant to the purpose of the TSCA test guideline, and to specify those provisions of the guidelines which the Agency believed must be made mandatory in order to ensure the integrity of any data produced by the test.
EPA sumarizes below, guideline-by-guideline, the changes the Agency made to the OPPTS harmonized test guideline in developing the TSCA test guideline.
A. Section 799.6755 TSCA Partition Coefficient (n-octanol/water), Shake Flask Method
1. EPA deleted references to FIFRA.
2. EPA made several grammatical changes.
3. EPA deleted references which were unavailable.
B. Section 799.6756 TSCA Partition Coefficient (n-octanol/water), Generator Column Method
1. EPA deleted references to FIFRA.
2. EPA made several grammatical changes.
3. EPA deleted references which were unavailable.
C. Section 799.6784 TSCA Water Solubility; Column Elution Method; Shake Flask Method
1. EPA deleted references to FIFRA.
2. EPA made several grammatical changes.
3. EPA deleted references which were unavailable.
D. Section 799.6786 TSCA, Water Solubility, Generator Column Method
1. EPA deleted references to FIFRA.
2. EPA made several grammatical changes.
3. EPA deleted references which were unavailable.
E. Section 799.9110 TSCA Acute Oral Toxicity
1. EPA clarified those provisions describing alternative acute testing procedures. EPA acknowledges that both the current OPPTS harmonized guideline and international consideration of acute toxicity guidelines are in a period of transition toward specifying reduced animal testing requirements.
2. EPA deleted references to FIFRA and discussions of pesticides.
3. EPA added “musts” to those requirements deemed critical to the successful production of scientifically-valid data for Agency risk assessment purposes.
F. Section 799.9120 TSCA Acute Dermal Toxicity
1. EPA deleted references to FIFRA and discussions of pesticides.
2. EPA added “musts” to those requirements deemed critical to the successful production of scientifically-valid data for Agency risk assessment purposes.
G. Section 799.9130 TSCA Acute Inhalation Toxicity
1. EPA deleted references to FIFRA and discussions of pesticides.
2. EPA added “musts” to those requirements deemed critical to the successful production of scientifically-valid data for Agency risk assessment purposes.
3. EPA revised and reorganized certain narrative sections for consistency with the comparable sections in the previously-promulgated 40 CFR 799.9135 (TSCA acute inhalation toxicity with histopathology).
H. Section 799.9305 TSCA Repeated Dose 28-day Oral Toxicity Study in Rodents
1. EPA deleted references to FIFRA and discussions of pesticides.
2. EPA made editorial changes to text to ensure consistency with the TSCA series of guidelines.
I. Section 799.9310 TSCA 90-day Oral Toxicity in Rodents
1. EPA deleted references to FIFRA and discussions of pesticides.
2. EPA added “musts” to those requirements deemed critical to the successful production of scientifically-valid data for Agency risk assessment purposes.
3. EPA removed the included neurotoxicity testing provisions in paragraphs (e)(8)(ii) through (e)(8)(v) because TSCA practice is to specify the more detailed neurotoxicity testing provisions of 40 CFR 799.9620.
J. Section 799.9325 TSCA 90-day Dermal Toxicity
1. EPA deleted references to FIFRA and discussions of pesticides.
2. EPA added “musts” to those requirements deemed critical to the successful production of scientifically-valid data for Agency risk assessment purposes.
3. EPA removed the included neurotoxicity testing provisions in paragraphs (e)(9)(ii) through (e)(9)(v) because TSCA practice is to specify the more detailed neurotoxicity testing provisions of 40 CFR 799.9620.
4. EPA deleted references which were unavailable.
K. Section 799.9355 TSCA Reproduction/Developmental Toxicity Screening Test
1. EPA deleted references to FIFRA and discussions of pesticides.
2. EPA made editorial changes to text to ensure consistency with the TSCA series of guidelines.
L. Section 799.9365 TSCA Combined Repeated Dose Toxicity Study With the Reproduction/Developmental Toxicity Screening Test
1. EPA deleted references to FIFRA and discussions of pesticides.
2. EPA made editorial changes to text to ensure consistency with the TSCA series of guidelines.
M. Section 799.9410 TSCA Chronic Toxicity
1. EPA deleted references to FIFRA and discussions of pesticides.
2. EPA removed recommendations for the use of particular non-rodent species.
N. Section 799.9430 TSCA Combined Chronic Toxicity/Carcinogenicity
1. EPA deleted references to FIFRA and discussions of pesticides.
2. EPA added “musts” to those requirements deemed critical to the successful production of scientifically-valid data for Agency risk assessment purposes.
3. EPA removed the included neurotoxicity testing provisions in paragraphs (e)(7)(ii)through (e)(7)(v) because TSCA practice is to specify the more detailed neurotoxicity testing provisions of 40 CFR 799.9620.
4. EPA deleted references which were unavailable.
5. EPA added a new provision (paragraph (e)(5)(ii)(J)) requiring that care be taken when the physical and chemical properties of the test substance show a low flash point or is otherwise known or thought to be explosive.
O. Section 799.9537 TSCA in vitro Mammalian Chromosome Aberration Test
1. EPA deleted references to FIFRA.
2. EPA made several provisions mandatory by specifying “must” instead of “should.” Start Printed Page 78750
3. EPA clarified the regulatory text in citing particular references in the standard.
P. Section 799.9630 TSCA Developmental Neurotoxicity
1. EPA deleted references to FIFRA.
2. EPA made several provisions mandatory by specifying “must” instead of “should.”
Q. Section 799.9748 TSCA Metabolism and Pharmacokinetics
1. EPA deleted references to FIFRA.
2. EPA made several provisions mandatory by specifying “must” instead of “should.”
V. Regulatory Assessment Requirements
A. Why is this Action Being Issued as a Final Rule?
EPA is publishing this action as a final rule without prior notice and an opportunity to comment because the Agency believes that providing notice and an opportunity to comment is unnecessary. The test guidelines codified in this document by themselves have no substantive effect on any person until and unless the test guidelines are incorporated in a test rule promulgated under TSCA section 4. Before any such test rule is promulgated, EPA will provide notice and an opportunity to comment on the incorporation of a particular test guideline into a specific test rule. In addition, the process for developing and amending the harmonized test guidelines includes broad public participation and extensive involvement of the scientific community. EPA therefore finds that there is “good cause” under section 553(b)(3)(B) of the Administrative Procedure Act (APA) (5 U.S.C. 553(b)(3)(B)) to codify these test guidelines without prior notice and comment, and that this rule may be made effective immediately, without a 30 day delay, pursuant to 5 U.S.C. 553(d)(3).
B. Do the Regulatory Assessment Requirements Apply to this Action?
No. As indicated previously, this final rule does not impose any requirements. It only incorporates test guidelines into the TSCA series of test guidelines that are published in the CFR and which would be considered for potential incorporation in a future test rule that would be proposed under TSCA section 4. At which time potentially affected entities are afforded an opportunity to comment on the incorporation of a particular test guideline into a specific test rule.
As such, this is not a “significant regulatory action” that requires review by the Office of Management and Budget (OMB) under Executive Order 12866, entitled Regulatory Planning and Review (58 FR 51735, October 4, 1993).
Since this action is not “economically significant” as defined by section 3(f) of Executive Order 12866, this action is not subject to Executive Order 13045, entitled Protection of Children from Environmental Health Risks and Safety Risks (62 FR 19885, April 23, 1997).
This action will not result in environmental justice related issues and does not, therefore, require special consideration under Executive Order 12898, entitled Federal Actions to Address Environmental Justice in Minority Populations and Low-Income Populations (59 FR 7629, February 16, 1994).
Since the Agency has made a “good cause” finding that this action is not subject to notice-and-comment requirements under the APA or any other statute (see Unit V.A.), this action is not subject to the regulatory flexibility provisions of the Regulatory Flexibility Act (RFA) (5 U.S.C. 601 et seq.), or to sections 202 and 205 of the Unfunded Mandates Reform Act of 1995 (UMRA) (Public Law 104-4). In addition, this action does not significantly or uniquely affect small governments or impose a significant intergovernmental mandate, as described in sections 203 and 204 of UMRA. Nor does this action significantly or uniquely affect the communities of tribal governments as specified by Executive Order 13084, entitled Consultation and Coordination with Indian Tribal Governments (63 FR 27655, May 10, 1998). This rule will not have substantial direct effects on the States, on the relationship between the national government and the States, or on the distribution of power and responsibilities among the various levels of government, as specified in Executive Order 13132, entitled Federalism (64 FR 43255, August 10, 1999).
This rule does not contain any information collection requirements that require review and approval by OMB pursuant to the Paperwork Reduction Act of 1995 (PRA) (44 U.S.C. 3501 et seq.).
In issuing this rule, EPA has taken the necessary steps to eliminate drafting errors and ambiguity, minimize potential litigation, and provide a clear legal standard for affected conduct, as required by section 3 of Executive Order 12988, entitled Civil Justice Reform (61 FR 4729, February 7, 1996).
EPA has also complied with Executive Order 12630, entitled Governmental Actions and Interference with Constitutionally Protected Property Rights (53 FR 8859, March 15, 1988), by examining the takings implications of this rule in accordance with the “Attorney General's Supplemental Guidelines for the Evaluation of Risk and Avoidance of Unanticipated Takings” issued under the Executive Order.
C. Are there Any Applicable Voluntary Consensus Standards?
No. Section 12(d) of the National Technology Transfer and Advancement Act of 1995 (NTTAA), Public Law 104-113, section 12(d) (15 U.S.C. 272 note), directs EPA to use voluntary consensus standards in its regulatory activities unless to do so would be inconsistent with applicable law or otherwise impractical. Voluntary consensus standards are technical standards (e.g., materials specifications, test methods, sampling procedures, business practices, etc.) that are developed or adopted by voluntary consensus standards bodies. The NTTAA requires EPA to provide an explanation to Congress, through OMB, when the Agency decides not to use available and applicable voluntary consensus standards when the NTTAA directs the Agency to do so.
As indicated earlier, this final rule does not impose any obligations on anyone until the test guidelines are incorporated in a test rule promulgated under TSCA section 4. Before any such test rule is promulgated, EPA will provide notice and an opportunity to comment on the incorporation of a particular test guideline into that specific test rule, including the availability of applicable voluntary consent standards.
In addition, although the NTTAA requirements do not specifically apply to the issuance of the harmonized test guidelines, EPA has sought comments on the availability of applicable voluntary consensus standards that should be considered during the development of future rules under TSCA. This allows the Agency to consider such standards during the development of the harmonized test guidelines, upon which the TSCA test guidelines are based.
VI. Submission to Congress and the Comptroller General
The Congressional Review Act, 5 U.S.C. 801 et seq., as added by the Small Business Regulatory Enforcement Fairness Act of 1996, generally provides that before a rule may take effect, the agency promulgating the rule must submit a rule report, which includes a Start Printed Page 78751copy of the rule, to each House of the Congress and to the Comptroller General of the United States. Section 808 allows the issuing agency to make a good cause finding that notice and public procedure is impracticable, unnecessary or contrary to the public interest. This determination must be supported by a brief statement. 5 U.S.C. 808(2). EPA has made such a good cause finding for this final rule, and established an effective date of December 15, 2000. Pursuant to 5 U.S.C. 808(2), this determination is supported by the brief statement in Unit V.A. EPA will submit a report containing this final rule and other required information to the U.S. Senate, the U.S. House of Representatives, and the Comptroller General of the United States prior to publication of the rule in the Federal Register. This is not a “major rule” as defined by 5 U.S.C. 804(2).
Start List of SubjectsList of Subjects in 40 CFR Part 799
- Environmental protection
- Chemicals
- Hazardous substances
- Health
- Reporting and recordkeeping requirements
End Signature Start Amendment PartDated: November 27, 2000.
Susan H. Wayland,
Acting Assistant Administrator for Prevention, Pesticides and Toxic Substances.
Therefore, 40 CFR part 799 is amended as follows:
End Amendment Part Start PartPART 799—[AMENDED]
End Part Start Amendment Part1. The authority citation for part 799 continues to read as follows:
End Amendment Part2. A new subpart E, consisting of §§ 799.6755 to 799.6786 is added to read as follows:
- 799.6755
- TSCA partition coefficient (n-octanol/water), shake flask method.
- 799.6756
- TSCA partition coefficient (n-octanol/water), generator column method.
- 799.6784
- TSCA water solubility: Column elution method; shake flask method.
- 799.6786
- TSCA water solubility: Generator column method.
Subpart E—Product Properties Test Guidelines Subpart E—Product Properties Test Guidelines
TSCA partition coefficient (n-octanol/water), shake flask method.(a) Scope—(1) Applicability. This section is intended to meet the testing requirements of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides and Toxics (OPPTS) harmonized test guideline 830.7550 (August 1996, final guideline). The source is available at the address in paragraph (f) of this section.
(b) Introductory information—(1) Prerequisites. Suitable analytical method, dissociation constant, water solubility, and hydrolysis (preliminary test).
(2) Coefficient of variation. The coefficient of variation on the mean values reported by the participants of the Organization for Economic Coopertion and Development (OECD) Laboratory Intercomparison Testing, Part I, 1979, appeared to be dependent on the chemicals tested; it ranges from 0.17 to 1.03.
(3) Qualifying statements. This method applies only to pure, water soluble substances which do not dissociate or associate, and which are not surface active. In order to use the partition coefficient (P) as a screening test for bioaccumulation, it should be ascertained that the impurities in the commercial product are of minor importance. Testing of P (n-octanol/water) cannot be used as a screening test in the case of organometallic compounds.
(4) Alternative methods. High-pressure liquid chromatography (HPLC) methods described in the references in paragraphs (f)(3), (f)(4), and (f)(5) of this section may be considered as an alternative test method.
(c) Method—(1) Introduction, purpose, scope, relevance, application, and limits of test. The P of a substance between water and a lipophilic solvent (n-octanol) is one model variable which may be used to describe the transfer of a substance from the aquatic environment into an organism and the potential bioaccumulation of the substance. Studies show a highly significant relationship between the P of different substances in the system water/n-octanol and their bioaccumulation in fish described in paragraph (f)(1) of this section.
(2) Definitions—Partition coefficient (P) is defined as the ratio of the equilibrium concentrations (Ci) of a dissolved substance in a two-phase system consisting of two largely immiscible solvents. The P therefore is the quotient of two concentrations and is usually given in the form of its logarithm to base 10 (log P). In this case n-octanol and water:
Equation 1:
(3) Reference substances. The reference substances need not be employed in all cases when investigating a new substance. They are provided primarily so that calibration of the method may be performed from time to time and to offer the chance to compare the results when another method is applied. The values presented in table 1 of this section are not necessarily representative of the results which can be obtained with this test method as they have been derived from an earlier version of the test guideline.
Table 1.—Data for Reference Substances
Tested substance1 Pow2 Di(2-ethylhexyl)phthalate (OECD) 1.3 × 105 (4.6 × 104 - 2.8 × 105) Hexachlorobenzene (OECD) 3.6 × 105 (1.1 × 105 - 8.3 × 105) o-Dichlorobenzene European Economic Community (EEC) 5.1 × 103 (1.5 × 103 - 2.3 × 104) Dibutyl phthalate (EEC) 1.3 × 104 (1.7 × 103 - 2.8 × 104) Trichloroethylene (OECD) 2.0 × 103 (5.2 x 102 - 3.7 x 103) Urea (OECD) 6.2 x 10-2 (2.0 x 10-2—2.4 x 10-1) 1 Substances not tested: Ethyl acetate, 4-methyl-2,4-pentanediol. 2 Total, mean, and range of mean values (in parentheses) submitted by the participants of the OECD or EEC Laboratory Intercomparison Testing. (4) Principle of the test method. In order to determine a P, equilibrium between all interacting components of the system must be achieved, and the concentrations of the substances dissolved in the two phases must be determined. A study of the literature on this subject indicates that there are many different techniques which can be Start Printed Page 78752used to solve this problem, i.e. the thorough mixing of the two phases followed by their separation in order to determine the equilibrium concentration for the substance being examined.
(5) Quality criteria—(i) Repeatability. In order to assure the precision of the P, duplicate determinations are to be made under three different test conditions, whereby the quantity of substance specified as well as the ratio of the solvent volumes may be varied. The determined values of the P expressed as their common logarithms should fall within a range of ± 0.3 log units.
(ii) Sensitivity. The sensitivity of the method is determined by the sensitivity of the analytical procedure. This should be sufficient to permit the assessment of values of Pow up to 105 when the concentration of the solute in either phase is not more than 0.01 mol/Liter (L). The substance being tested must not be water insoluble (mass concentration r ≤ 10-6 gram (g)/L.
(iii) Specificity. The Nernst Partition Law applies only at constant temperature, pressure, and pH for dilute solutions. It strictly applies to a pure substance dispersed between two pure solvents. If several different solutes occur in one or both phases at the same time, this may affect the results. Dissociation or association of the dissolved molecules result in deviations from the Nernst Partition Law. Such deviations are indicated by the fact that the P becomes dependent upon the concentration of the solution. Because of the multiple equilibria involved, this test guideline should not be applied to ionizable compounds without corrections being made. The use of buffer solutions in place of water should be considered for such compounds.
(iv) Possibility of standardization. This method can be standardized.
(d) Description of the test procedure—(1) Preparations: Preliminary estimate of the P. The size of the P can be estimated either by means of calculation or by use of published solubilities of the test substance in the pure solvents. Alternatively, it may be roughly determined by performing a simplified preliminary test. For this:
Equation 2:
(2) Preparation of the solvents—(i) n-Octanol. The determination of the P should be carried out with analytical grade n-octanol. Inorganic contaminants can be removed from commercial n-octanol by washing with acid and base, drying, and distilling. More sophisticated methods will be required to separate the n-octanol from organic contaminants with similar vapor pressure if they are present.
(ii) Water. Distilled water or water twice-distilled from glass or quartz apparatus should be employed. Water taken directly from an ion exchanger should not be used.
(iii) Presaturation of the solvents. Before a P is determined, the phases of the solvent system are mutually saturated by shaking at the temperature of the experiment. For doing this, it is practical to shake two large stock bottles of purified n-octanol or distilled water each with a sufficient quantity of the other solvent for 24 hours on a mechanical shaker, and then to let them stand long enough to allow the phases to separate and to achieve a saturation state.
(3) Preparation for the test. The entire volume of the two-phase system should nearly fill the test vessel. This will help prevent loss of material due to volatilization. The volume ratio and quantities of substance to be used are fixed by the following:
(i) The preliminary assessment of the P as discussed in paragraph (d)(1) of this section).
(ii) The minimum quantity of test substance required for the analytical procedure.
(iii) The limitation of a maximum concentration in either phase of 0.01 mol/L.
(iv) Three tests are carried out. In the first, the calculated volume ratio is added; in the second, twice the volume of n-octanol is added; and in the third, half the volume of n-octanol is added.
(4) Test substance. The test substance should be the purest available. For a material balance during the test a stock solution is prepared in n-octanol with a mass concentration between 1 and 100 milligram/milliliter (mg/mL). The actual mass concentration of this stock solution should be precisely determined before it is employed in the determination of the P. This solution should be stored under stable conditions.
(5) Test conditions. The test temperature should be kept constant (± 1 °C) and lie in the range of 20-25 °C.
(6) Performance of the test—(i) Establishment of the partition equilibrium. Duplicate test vessels containing the required, accurately measured amounts of the two solvents together with the necessary quantity of the stock solution should be prepared for each of the test conditions. The n-octanol parts should be measured by volume. The test vessels should either be placed in a suitable shaker or shaken by hand. A recommended method is to rotate the centrifuge tube quickly through 180° about its transverse axis so that any trapped air rises through the two phases. Experience has shown that 50 such rotations are usually sufficient for the establishment of the partition equilibrium. To be certain, 100 rotations in 5 minutes are recommended.
(ii) Phase separation. In order to separate the phases, centrifugation of the mixture should be carried out. This should be done in a laboratory centrifuge maintained at room temperature, or, if a non-temperature-controlled centrifuge is used, the centrifuge tubes should be reequilibrated at the test temperature for at least 1 hour before analysis.
(7) Analysis. (i) For the determination of the P, it is necessary to analyze the concentrations of the test substance in both phases. This may be done by taking an aliquot of each of the two phases from each tube for each test condition and analyzing them by the chosen procedure. The total quantity of substances present in both phases should be calculated and compared with the quantity of the substance originally introduced.
(ii) The aqueous phase should be sampled by the following procedure to minimize the risk of including traces of the n-octanol: A glass syringe with a removable needle should be used to sample the water phase. The syringe should initially be partially filled with air. Air should be gently expelled while inserting the needle through the n-octanol layer. An adequate volume of aqueous phase is withdrawn into the syringe. The syringe is quickly removed from the solution and the needle detached. The contents of the syringe may then be used as the aqueous sample.
(iii) The concentration in the two-separated phases should preferably be determined by a substance-specific method. Examples of physical-chemical Start Printed Page 78753determinations which may be appropriate are:
(A) Photometric methods.
(B) Gas chromatography.
(C) HPLC.
(D) Back-extraction of the aqueous phase and subsequent gas chromatography.
(e) Data and reporting—(1) Treatment of results. The reliability of the determined values of P can be tested by comparison of the means of the duplicate determinations with the overall mean.
(2) Test report. The following should be included in the report:
(i) Name of the substance, including its purity.
(ii) Temperature of the determination.
(iii) The preliminary estimate of the P and its manner of determination.
(iv) Data on the analytical procedures used in determining concentrations.
(v) The measured concentrations in both phases for each determination. This means that a total of 12 concentrations must be reported.
(vi) The weight of the test substance, the volume of each phase employed in each test vessel, and the total calculated amount of test substance present in each phase after equilibration.
(vii) The calculated values of the P and the mean should be reported for each set of test conditions as should the mean for all determinations. If there is a suggestion of concentration dependency of the P, this should be noted in the report.
(viii) The standard deviation of individual P values about their mean should be reported.
(ix) The mean P from all determinations should also be expressed as its logarithm (base 10).
(f) References. For additional background information on this test guideline, the following references should be consulted. These references are available from the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., SW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, excluding legal holidays.
(1) Neely, W.B. et al. Partition Coefficients to Measure Bioconcentration Potential of Organic Chemicals in Fish. Environmental Science and Technology 8:1113 (1974).
(2) Leo, A. et al. Partition Coefficients and Their Uses. Chemical Reviews 71:525 (1971).
(3) Miyake, K. and H. Terada, Direct measurements of partition coefficients in an octanol-water system. Journal of Chromatography 157:386 (1978).
(4) Veith G.D. and R.T. Morris, A Rapid Method for Estimating Log P for Organic Chemicals, EPA-600/3-78-049 (1978).
(5) Mirrless, M.S. et al., Direct measurement of octanol-water partition coefficient by high pressure liquid chromatography. Journal of Medicinal Chemistry 19:615 (1976).
(6) EPA Draft Guidance of September 8, 1978 (F-16).
(7) Konemann H. et al. Determination of log Poct values of chlorosubstituted benzenes, toluenes, and anilines by high performance liquid chromatography on ODS silica, Journal of Chromatography 178:559 (1979).
(8) Organization for Economic Cooperation and Development, Guidelines for The Testing of Chemicals, OECD 107, Partition Coefficient (n-octanol/water) (Shake Flask Method, Adopted 27 July 1995), OECD, Paris, France.
TSCA partition coefficient (n-octanol/water), generator column method.(a) Scope—(1) Applicability. This section is intended to meet the testing requirements of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Source. The source material used in developing this TSCA test guideline is the Office of Pollution Prevention, Pesticides and Toxic Substances (OPPTS) harmonized test guideline 830.7560 (August 1996, final guideline). This source is available at the address in paragraph (e) of this section.
(b) (1) Purpose. (i) The measurement and estimation of the n-octanol/water partition coefficient (Kow), has become the cornerstone of a myriad of structure-activity relationships (SAR) property. The coefficient has been used extensively for correlating structural changes in drugs with changes observed in biological, biochemical, or toxic effects. These correlations are then used to predict the effect of a new drug for which a Kow could be measured.
(ii) In the study of the environmental fate of organic chemicals, the Kow has become a key parameter. Kow is correlated to water solubility, soil/sediment sorption coefficient, and bioconcentration and is important to SAR.
(iii) Of the three properties that can be estimated from Kow, water solubility is the most important because it affects both the fate and transport of chemicals. For example, highly soluble chemicals become quickly distributed by the hydrologic cycle, have low-sorption coefficients for soils and sediments, and tend to be more easily degraded by microorganisms. In addition, chemical transformation processes such as hydrolysis, direct photolysis, and indirect photolysis (oxidation) tend to occur more readily if a compound is soluble.
(iv) Direct correlations between Kow and both the soil/sediment sorption coefficient and the bioconcentration factor are to be expected. In these cases, compounds that are more soluble in n-octanol (more hydrophobic and lipophilic) would be expected to partition out of the water and into the organic portion of soils/sediments and into lipophilic tissue. The relationship between Kow and the bioconcentration factor, are the principal means of estimating bioconcentration factors. This relationship is discussed in the reference listed in paragraph (e)(14) of this section. These factors are then used to predict the potential for a chemical to accumulate in living tissue.
(v) This section describes a method for determining the Kow based on the dynamic coupled column liquid chromatographic (DCCLC) technique, a technique commonly referred to as the generator column method. The method described herein can be used in place of the standard shake-flask method specified in § 799.6755 for compounds with a log10 Kow greater than 1.0.
(2) Definitions. The following definitions apply to this section.
Extractor column is used to extract the solute from the aqueous solution produced by the generator column. After extraction onto a bonded chromatographic support, the solute is eluted with a solvent/water mixture and subsequently analyzed by high-performance liquid chromatography (HPLC), gas chromatography (GC), or any other analytical procedure. A detailed description of the preparation of the extractor column is given in paragraph (c)(1)(i) of this section.
Generator column is used to partition the test substance between the n-octanol and water phases. The column in figure 1 in paragraph (c)(1)(i)(A)(2) of this section is packed with a solid support and is coated with the test substance at a fixed concentration in n-octanol. The test substance is eluted from the column with water and the aqueous solution leaving the column represents the equilibrium concentration of the test substance that has partitioned from the n-octanol phase into the water phase. Preparation of the generator column is described in paragraph (c)(1)(i) of this section.
n-Octanol/water partition coefficient (Kow) is defined as the ratio of the molar concentrations of a chemical in n-octanol and water, in dilute solution. The coefficient Kow is a constant for a given chemical at a given temperature. Since Kow is the ratio of two molar concentrations, it is a dimensionless Start Printed Page 78754quantity. Sometimes Kow is reported as the decadic logarithm (log10 Kow). In this equation, Coctanol and Cwater are the molar concentration of the solute in n-octanol and water, respectively, at a given temperature. This test procedure determines Kow at 25 ± 0.05 °C. The mathematical statement of Kow is:
Equation 1:
Response factor (RF) is the solute concentration required to give a one unit area chromatographic peak or one unit output from the HPLC recording integrator at a particular recorder and detector attenuation. The factor is required to convert from units of area to units of concentration. The determination of the RF is given in paragraph (c)(3)(iii)(C)(2) of this section.
Sample loop is a 1/16 inch (in) outside diameter (O.D.) (1.6 millimeter (mm)) stainless steel tube with an internal volume between 20 and 50 mL. The loop is attached to the sample injection valve of the HPLC and is used to inject standard solutions into the mobile phase of the HPLC when determining the RF for the recording integrator. The exact volume of the loop must be determined as described in paragraph (c)(3)(iii)(C)(1) of this section when the HPLC method is used.
(3) Principle of the test method. (i) This test method is based on the DCCLC technique for determining the aqueous solubility of organic compounds. The development of this test method is described in the references listed in paragraphs (e)(6), (e)(12), and (e)(19) of this section. The DCCLC technique utilizes a generator column, extractor column, and HPLC coupled or interconnected to provide a continuous closed-flow system. Aqueous solutions of the test compound are produced by pumping water through the generator column that is packed with a solid support coated with an approximately 1.0% weight/weight (w/w) solution of the compound in n-octanol. The aqueous solution leaving the column represents the equilibrium concentration of the test chemical which has partitioned from the n-octanol phase into the water phase. The compound is extracted from the aqueous solution onto an extractor column, then eluted from the extractor column with a solvent/water mixture and subsequently analyzed by HPLC using a variable wavelength ultraviolet (UV) absorption detector operating at a suitable wavelength. Chromatogram peaks are recorded and integrated using a recording integrator. The concentration of the compound in the effluent from the generator column is determined from the mass of the compound (solute) extracted from a measured volume of water (solvent). The Kow is calculated from the ratio of the molar concentration of the solute in the 1.0% (w/w) n-octanol and molar concentration of the solute in water as determined using the generator column technique.
(ii) Since the HPLC method is only applicable to compounds that absorb in the UV, an alternate GC method, or any other reliable quantitative procedure must be used for those compounds that do not absorb in the UV. In the GC method the saturated solutions produced in the generator column are extracted using an appropriate organic solvent that is subsequently injected into the GC, or any other suitable analytical device, for analysis of the test compound.
(4) Reference chemicals. (i) Columns 2, 3, 4, and 5 of table 1 in paragraph (b)(4)(ii) of this section list the experimental values of the decadic logarithm of the n-octanol/water partition coefficient (log10 Kow) at 25 °C for a number of organic chemicals as obtained from the scientific literature. These values were obtained by any one of the following experimental methods: Shake-flask; generator column; reverse-phase HPLC; or reverse-phase thin-layer chromatography, as indicated in the footnotes following each literature citation. The estimation method of Hawker and Connell as described in paragraph (e)(8) of this section, correlates log10 Kow with the total surface area of the molecule and was used to estimate log10 Kow for biphenyl and the chlorinated biphenyls. These estimated values are listed in column 7 of table 1 in paragraph (b)(4)(ii) of this section. Recommended values of log10 Kow were obtained by critically analyzing the available experimental and estimated values and averaging the best data. These recommended values are listed in column 8 of table 1 in paragraph (b)(4)(ii) of this section.
(ii) The recommended values listed in table 1 of this section have been provided primarily so that the generator column method can be calibrated and to allow the chemical laboratory the opportunity to compare its results with these values. The testing laboratory has the option of choosing its reference chemicals, but references must be given to establish the validity of the measured values of log10 Kow.
Start Printed Page 78756Table 1.—n-Octanol/Water Partition Coefficient at 25 °C for Some Reference Compounds
Chemical Experimental log10 Kow Estimated log10 Kow Recommended log10 Kow Hansch and Leo1 Generator Column Method Banerjee2 Other values Hansch and Leo3 Hawker and Connell4 Ethyl acetate 0.73, 0.66 5 0.68 — — 0.671 — 17 0.685 1-Butanol 0.88, 0.89, 0.32, 0.88 5 0.785 — — 0.823 — 23 0.852 1-Pentanol 1.28, 1.40 5 1,53 — — 1.35 — 17 1.39 Nitrobenzene 1.85, 1.88, 1.79 5 1.85 1.83 6 1.82 1.89 — 17 1.84 Benzene 2.15, 2.13 — 2.12 — 2.14 — 17 2.14 Trichloroethylene 2.29 5 2.53 2.42 — 2.27 — 17 2.38 Chlorobenzene 2.84, 2.46 7 2.98 — 8 2.84 2.86 — 18 2.80 o-Dichlorobenzene 3.38 7 3.38 3.40 8 3.38 3.57 — 17 3.42 n- Propylbenzene 3.66, 3.66, 3.68, 3.57 5 3.69 — — 3.85 — 17 3.69 Biphenyl 3.95, 4.17, 4.09, 4.04 7 3.67,9 3.89,10 3.79 4.04 6 3.75 4.03 4.09 17 3.96 Start Printed Page 78755 2-Chlorobiphenyl — 7 4.50,9 4.38 — 10 3.90,11 3.75,12 4.59,13 4.54 — 4.99 19 4.49 1,2,3,5-Tetrachlorobenzene — 7 4.65 4.46 — 4.99 — 17 4.70 2,2′-Dichlorobiphenyl — 9 4.90 — 9 4.90,10 3.63,11 3.55,14 4.51,15 5.02 — 4.65 20 4.80 Pentachlorobenzene — 7 5.03 4.94 — 5.71 — 24 4.99 2,4,5-Trichlorobiphenyl — 7 5.51,9 5.81 — 10 5.67,10 5.86,15 5.77 — 5.60 17 5.70 2,3,4,5-Tetrachlorobiphenyl — 4 6.18,7 5.72 — — — 6.04 17 5.98 2,2′,4,5,5′-Pentachlorobi-phenyl 6.11 9 6.50,7 5.92 — 13 6.11,12 6.85 — 6.38 17 6.31 2,2′,3,3′,6,6′-Hexachloro-biphenyl — 4 5.76,7 6.63,9 6.81 — — — 6.22 17 6.36 2,2′,3,3′,4,4′,6-Heptachlorobiphenyl — 7 6.68 — — — 7.11 17 6.90 2,2′,3,3′,5,5′,6,6′-Octachlorobiphenyl — 7 7.11,9 7.14 — 12 8.42 — 7.24 21 7.16 2,2′,3,3′,4, 4′,5,6,6′-Nona-chlorobiphenyl — 4 7.52 — — — 7.74 17 7.63 2,2′,3,3′,4, 5,5′6,6′-Nona-chlorobiphenyl — 7 8.16 — — — 7.71 17 7.94 Decachlorobiphenyl — 7 8.26,9 8.20 — 12 9.60 — 8.18 22 8.21 1 Hansch and Leo (1979). Shake-flask method in paragraph (e)(8) of this section. 2 Banerjee, Yalkowski, and Valvani (1980). Shake-flask method in paragraph (e)(1) of this section. 3 Hansch and Leo (1984). Estimates log10 Kow using the CLogP3 computer program in paragraph (e)(9) of this section. 4 Hawker and Connell (1988). Generator column method and an estimation method correlating log10 Kow with the total surface area of the molecule in paragraph (e)(8) of this section. 5 Tewari et al. (1982). Generator column method in paragraph (e)(14) of this section. 6 Veith, Austin, and Morris (1979). Reverse-phase HPLC method in paragraph (e)(16) of this section. 7 Miller et al. (1984). Generator column method in paragraph (e)(11) of this section. 8 Chiou and Schmedding (1982). Shake-flask method in paragraph (e)(4) of this section. 9 Woodburn, Doucette, and Andren (1984). Generator column method in paragraph (e)(19) of this section. 10 Rapaport and Eisenreich (1984). Reverse-phase HPLC method in paragraph (e)(13) of this section. 11 Woodburn (1982). Reverse-phase HPLC method in paragraph (e)(18) of this section. 12 Bruggemann, Van der Steen, and Hutzinger (1978). Shake-flask method in paragraph (e)(2) of this section. 13 Tulp and Hutzinger (1978). Shake-flask method in paragraph (e)(15) of this section. 14 Chiou, Porter, and Schmedding (1983). Shake-flask method in paragraph (e)(5) of this section. 15 Bruggemann, Van Der Steen , and Hutzinger (1982). Reverse-phase thin-layer chromatography in paragraph (e)(2) of this section. 16 Chiou et al. (1977). Shake-flask method in paragraph (e)(3) of this section. 17 Average value using all the data. 18 Average value using all the data except the datum point 2.46. 19 Average value using all the data except the data points 3.90 and 3.75. 20 Average value using all the data except the data points 3.63 and 3.55. 21 Average value using all the data except the datum point 8.42. 22 Average value using all the data except the datum point 9.60. 23 Average value using all the data except the datum point 0.32. 24 Average value using all the data excluding the estimated datum point 5.71. (5) Applicability and specificity. The test guideline is designed to determine the Kow of solid or liquid organic chemicals in the range log10 Kow 1.0 to ≤6.0 (10 to ≤106).
(c) Test procedure—(1) Test conditions—(i) Special laboratory equipment—(A)(1) Generator column. Either of two different methods for connecting to the generator column shall be used depending on whether the eluted aqueous phase is analyzed by HPLC (Procedure A, as described in paragraph (c)(3)(iii) of this section) or by solvent extraction followed by GC analysis, or any other reliable method of solvent extract (Procedure B, as described in paragraph (c)(3)(iv) of this section).
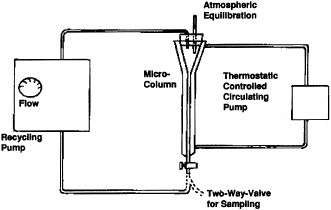
(2)(i) The design of the generator column is shown in the following figure 1:
(ii) The column consists of a 6 mm (1/4 in) O.D. pyrex tube joined to a short enlarged section of 9 mm pyrex tubing which in turn is connected to another section of 6 mm (1/4 in) O.D. pyrex tubing. Connections to the inlet teflon tubing (1/8 in O.D.) and to the outlet stainless steel tubing (1/16 in O.D.) are made by means of stainless steel fittings with teflon ferrules. The column is enclosed in a water jacket for temperature control as shown in the following figure 2:
Figure 2—Setup Showing Generator Column Enclosed in a Water Jacket and Overall Arrangement of the Apparatus Used in GC Method
(B) Constant temperature bath with circulation pump-bath and capable of controlling temperature to 25 ±0.05 °C. (Procedures A and B, as described in paragraphs (c)(3)(iii) and (c)(3)(iv) of this section, respectively).
(C) HPLC equipped with a variable wavelength UV absorption detector operating at a suitable wavelength and a recording integrator (Procedure A, as described in paragraph (c)(3)(iii) of this section).
(D) Extractor column—6.6 × 0.6 centimeter (cm) stainless steel tube with end fittings containing 5 micron frits filled with a superficially porous phase packing (such as Bondapack C18 Corasil: Waters Associates) (Procedure A, as described in paragraph (c)(3)(iii) of this section).
(E) Two 6-port high-pressure rotary switching valves (Procedure A, as described in paragraph (c)(3)(iii) of this section).
(F) Collection vessel—8 × 3/4 in section of pyrex tubing with a flat bottom connected to a short section of 3/8 in O.D. borosilicate glass tubing. The collecting vessel is sealed with a 3/8 in teflon cap fitting (Procedure B, as described in paragraph (c)(3)(iv) of this section).
(G) GC, or any other reliable analytic equipment, equipped with a detector sensitive to the solute of interest (Procedure B, as described in paragraph (c)(3)(iv) of this section).
(ii) Purity of n-octanol and water. Purified n-octanol, described in paragraph (c)(2)(i) of this section, and water meeting appropriate American Society for Testing and Materials Type II standards, or an equivalent grade, are recommended to minimize the effects of dissolved salts and other impurities. An ASTM Type II water standard is presented in the reference listed in paragraph (e)(20) of this section).
(iii) Purity of solvents. It is important that all solvents used in this method be reagent or HPLC grade and contain no impurities which could interfere with the determination of the test compound.
(iv) Reference compounds. In order to ensure that the HPLC system is working properly, at least two of the reference compounds listed in table 1 in paragraph (b)(4)(ii) of this section should be run. Reference compounds shall be reagent or HPLC grade to avoid interference by impurities.
(2) Preparation of reagents and solutions—(i) n-Octanol and water. Very pure n-octanol can be obtained as follows: Wash pure n-octanol (minimum 98% pure) sequentially with 0.1N H2 SO4, with 0.1N NaOH, then with distilled water until neutral. Dry the n-octanol with magnesium sulfate and distill twice in a good distillation column under reduced pressure [b.p. about 80°C at 0.27 kPa (2 torr)]. The n-octanol produced should be at least 99.9% pure. Alternatively, a grade equivalent to Fisher Scientific Co. No. A-402 “Certified Octanol-1” can be used. Reagent-grade water shall be used throughout the test procedure, such as ASTM Type II water, or an equivalent grade, as described in paragraph (c)(1)(ii) of this section.
(ii) Presaturated water. Prepare presaturated water with n-octanol to minimize the depletion of n-octanol from the column when measuring the Kow of a test chemical. This is very important when the test chemical is lipophilic and the log10 Kow ≤4.
(3) Performance of the test. Initially, an approximately 1.0% (w/w) solution of the test substance in n-octanol is prepared. Precise measurement of the solute concentration in this solution is required for the Kow calculation. Subsequently, the 1.0% (w/w) solution is coated on the generator column and using either Procedure A or Procedure B as described in paragraphs (c)(3)(iii) and (c)(3)(iv) of this section, the molar concentration of the test substance in reagent-grade water is determined.
(i) Test solution. The test solution consists of an approximately 1.0% (w/w) solution of the test substance in n-octanol. A sufficient quantity (about 10-20 milliliter (mL)) of the test solution should be prepared to coat the generator column. The solution is prepared by accurately weighing out, using a tared Start Printed Page 78757bottle, quantities of both the test substance and n-octanol required to make a 1.0% (w/w) solution. When the weights are measured precisely (to the nearest 0.1 milligram (mg)), knowing the density of n-octanol (0.827 gram (g)/mL at 25 °C), then the molar concentration of the test substance in the n-octanol is sufficiently accurate for the purposes of the test procedure. If desired, however, a separate analytical determination (e.g., by GC, or any other reliable analytical method) may be used to check the concentration in the test solution. If storage is required, the test solution should be kept stoppered to prevent volatilization of the test chemical.
(ii) Test procedures. Prior to the determination of the Kow of the test chemical, two procedures shall be followed:
(A) The saturated aqueous solution leaving the generator column shall be tested for the presence of an emulsion, using a Tyndall procedure (i.e. light scattering). If colloids are present, they must be removed prior to injection into the extractor column by lowering the flow rate of water.
(B) The efficiency of removal of the solute (the test chemical) by solvent extraction from the extractor column shall be determined and used in the determination of the Kow of the test chemical.
(iii) Procedure A—HPLC method. (A) Procedure A covers the determination of the aqueous solubility of compounds which absorb in the UV. Two reciprocating piston pumps deliver the mobile phase (water or solvent/water mixture) through two 6-port high-pressure rotary valves and a 30 x 0.6 cm C18 analytical column to a UV absorption detector operating at a suitable wavelength. Chromatogram peaks are recorded and integrated with a recording integrator. One of the 6-port valves is the sample injection valve used for injecting samples of standard solutions of the solute in an appropriate concentration for determining RFs or standard solutions of basic chromate for determining the sample-loop volume. The other 6-port valve in the system serves as a switching valve for the extractor column which is used to remove solute from the aqueous solutions. The HPLC analytical system is shown schematically in the following figure 3:
Figure 3—Schematic of HPLC—Generator Column Flow System
(B) The general procedure for analyzing the aqueous phase after equilibration is as follows; a detailed procedure is given in paragraph (c)(3)(iii)(C)(4) of this section:
(1) Direct the aqueous solution from the generator column to “Waste” in figure 3 in paragraph (c)(3)(iii)(A) of this section with the switching valve in the inject position in order to equilibrate internal surfaces with the solution, thus insuring that the analyzed sample would not be depleted by solute adsorption on surfaces upstream from the valve.
(2) At the same time, water is pumped from the HPLC pumps in order to displace the solvent from the extractor column.
(3) The switching valve is next changed to the load position to divert a sample of the solution from the generator column through the extractor column, and the liquid leaving the extractor column is collected in a tared weighing bottle. During this extraction step, the HPLC mobile phase is changed to a solvent/water mixture to condition the analytical column.
(4) After the desired volume of sample is extracted, the switching valve is returned to the inject position for elution from the extractor column and analysis. Assuming that all of the solute was adsorbed by the extractor column during the extraction step, the chromatographic peak represents all of the solute in the extracted sample, provided that the extraction efficiency is 100%. If the extraction efficiency is less than 100%, then the extraction efficiency shall be measured and used to determine the actual amount of the solute extracted.
(5) The solute concentration in the aqueous phase is calculated from the peak area, the weight of the extracted liquid collected in the weighing bottle, the extraction efficiency, and the RF.
(C)(1) Determination of the sample-loop volume. Accurate measurement of the sample loop may be accomplished by using a spectrophotometric method such as the one described in the reference listed in paragraph (e)(6) of this section. For this method, measure absorbance, Aloop, at 373 nanometers (nm) for at least three solutions, each of which is prepared by collecting from the sample valve an appropriate number, n, of loopfuls of an aqueous stock solution of K2 CrO4 (1.3% by weight) and diluting to 50 mL with 0.2% KOH. (For a 20 mL loop, use n = 5; for a 50 mL loop, use n = 2.) Also measure the absorbance, Astock, of the same stock solution after diluting 1:500 with 0.2% KOH. Calculate the loop volume to the nearest 0.1 mL using the relation:
Equation 2:
(2) Determination of the RF. (i) For all determinations adjust the mobile phase solvent/water ratio and flow rate to obtain a reasonable retention time on the HPLC column. For example, typical concentrations of organic solvent in the mobile phase range from 50 to 100% while flow rates range from 1 to 3 mL/minutes (min); these conditions often give a 3 to 5 min retention time.
(ii) Prepare standard solutions of known concentrations of the solute in a suitable solvent. Concentrations must Start Printed Page 78758give a recorder response within the maximum response of the detector. Inject samples of each standard solution into the HPLC system using the calibrated sample loop. Obtain an average peak area from at least three injections of each standard sample at a set detector absorbance unit full scale (AUFS), i.e., at the same absorbance scale attenuation setting.
(iii) Calculate the RF from the following equation:
Equation 3:
(3) Loading of the generator column. (i) The design of the generator column was described in paragraph (c)(1)(i) of this section and is shown in figure 1 in paragraph (c)(1)(i)(A)(2)(i) of this section. To pack the column, a plug of silanized glass wool is inserted into one end of the 6 mm pyrex tubing. Silanized diatomaceous silica support (about 0.5g of 100-120 mesh Chromosorb W chromatographic support material) is poured into the tube with tapping and retained with a second plug of silanized glass wool.
(ii) The column is loaded by pulling the test solution through the dry support with gentle suction and then allowing the excess solution to drain out. After loading the column, draw water up through the column to remove any entrapped air.
(4) Analysis of the solute. Use the following procedure to collect and analyze the solute:
(i) With the switching valve in figure 3 in paragraph (c)(3)(iii)(A) of this section in the inject position (i.e., water to waste), pump water through the generator column at a flow rate of approximately 1 mL/min for approximately 15 min to bring the system into equilibrium. Pump water to the generator column by means of a minipump or pressurized water reservoir as shown in the following figure 4:
Figure 4—Water Reservoir for GC Method
(ii) Flush out the organic solvent that remains in the system from previous runs by changing the mobile phase to 100% H2 O and allowing the water to reach the HPLC detector, as indicated by a negative reading. As soon as this occurs, place a 25 mL weighing bottle (weighed to the nearest mg) at the waste position and immediately turn the switching valve to the load position.
(iii) Collect an amount of water from the generator column (as determined by trial and error) in the weighing bottle, corresponding to the amount of solute adsorbed by the extractor column that gives a reasonable detector response. During this extraction step, switch back to the original HPLC mobile phase composition, i.e., solvent/water mixture, to condition the HPLC analytical column.
(iv) After the desired volume of sample has been extracted, turn the switching valve back to the inject position in figure 3 in paragraph (c)(3)(iii)(A) of this section. As soon as the switching valve is turned to the inject position, remove the weighing bottle, cap it and replace it with the waste container; at the same time turn on the recording integrator. The solvent/water mobile phase will elute the solute from the extractor column and transfer the solute to the HPLC analytical column.
(v) Determine the weight of water collected to the nearest mg and record the corresponding peak area. Using the same AUFS setting repeat the analysis of the solute at least two more times and determine the average ratio of peak area to grams of water collected. In this equation, S = solubility (M), RF = response factor, Vloop = sample-loop volume (L), and R = ratio of area to grams of water. Calculate the solute solubility in water using the following equation:
Equation 4:
(iv) Procedure B—GC Method. In the GC method, or any other reliable quantitative method, aqueous solutions from the generator column enter a collecting vessel in figure 2 in paragraph (c)(1)(i)(A)(2)(ii) of this section containing a known weight of extracting solvent which is immiscible in water. The outlet of the generator column is positioned such that the aqueous phase always enters below the extracting solvent. After the aqueous phase is collected, the collecting vessel is stoppered and the quantity of aqueous phase is determined by weighing. The solvent and the aqueous phase are equilibrated by slowly rotating the collecting vessel. A small amount of the extracting solvent is then removed and injected into a GC equipped with an appropriate detector. The solute concentration in the aqueous phase is determined from a calibration curve constructed using known concentrations of the solute. The extraction efficiency of the solvent shall be determined in a separate set of experiments.
(A) Determination of calibration curve. (1) Prepare solute standard solutions of concentrations covering the expected range of the solute solubility. Select a column and optimum GC operating conditions for resolution between the solute and solvent and the Start Printed Page 78759solute and extracting solvent. Inject a known volume of each standard solution into the injection port of the GC. For each standard solution determine the average of the ratio R of peak area to volume (in mL) for the chromatographic peak of interest from at least three separate injections.
(2) After running all the standard solutions, determine the coefficients, a and b, using linear regression analysis on the equation of concentration (C) vs. R in the form:
Equation 5:
(B) Loading of the generator column. The generator column is packed and loaded with solute in the same manner as for the HPLC method in paragraph (c)(3)(iii) of this section. As shown in figure 2 in paragraph (c)(1)(i)(A)(2)(ii) of this section, attach approximately 20 cm of straight stainless steel tubing to the bottom of the generator column. Connect the top of the generator column to a water reservoir in figure 4 in paragraph (c)(3)(iii)(C)(4)(i) of this section using teflon tubing. Use air or nitrogen pressure (5 PSI) from an air or nitrogen cylinder to force water from the reservoir through the column. Collect water in an Erlenmeyer flask for approximately 15 min while the solute concentration in water equilibrates; longer time may be required for less soluble compounds.
(C) Collection and extraction of the solute. During the equilibration time, add a known weight of extracting solvent to a collection vessel which can be capped. The extracting solvent should cover the bottom of the collection vessel to a depth sufficient to submerge the collecting tube but still maintain 100:1 water/solvent ratio. Record the weight (to the nearest mg) of a collection vessel with cap and extracting solvent. Place the collection vessel under the generator column so that water from the collecting tube enters below the level of the extracting solvent in figure 2 in paragraph (c)(1)(i)(A)(2)(ii) of this section. When the collection vessel is filled, remove it from under the generator column, replace cap, and weigh the filled vessel. Determine the weight of water collected. Before analyzing for the solute, gently rotate the collection vessel contents for approximately 30 min, controlling the rate of rotation so as not to form an emulsion; rotating the flask end over end five times per minute is sufficient. The extraction efficiency of the solvent shall be determined in a separate set of experiments.
(D) Analysis of the solute. (1) After rotating, allow the collection vessel to stand for approximately 30 min; then remove a known volume of the extracting solvent from the vessel using a microliter syringe and inject it into the GC. Record the ratio of peak area to volume injected and, from the regression equation of the calibration line, determine the concentration of solute in the extracting solvent. If the extraction efficiency is not 100%, the measured extraction efficiency shall be used to obtain the correct concentration of solute extracted. In this equation, Ces is the molar concentration of solute in extracting solvent, dH2O and des are the densities in grams per milliliter of water and extracting solvent, respectively, and ges and gH2O are the grams of extracting solvent and water, respectively, contained in the collection vessels. The molar concentration of solute in water C(M) is determined from the following equation:
Equation 6:
(2) Make replicate injections from each collecting vessel to determine the average solute concentration in water for each vessel. To make sure the generator column has reached equilibrium, run at least two additional (for a total of three) collection vessels and analyze the extracted solute as described in paragraph (c)(3)(iv)(D)(1) of this section. Calculate C(M) from the average solute concentration in the three vessels.
(3) If another analytical method is used in place of the GC, then Procedure B, as described in paragraph (c)(3)(iv) of this section, shall be modified and the new analytical procedure shall be used to determine quantitatively the amount of solute extracted in the extraction solvent.
(v) Analysis of reference compounds. Prior to analyzing the test solution, make duplicate runs on at least two of the reference compounds listed in table 1 in paragraph (b)(4)(ii) of this section. When using the reference compounds, follow the same procedure previously described for preparing the test solution and running the test. If the average value obtained for each compound is within 0.1 log unit of the reference value, then the test procedure and HPLC system are functioning properly; if not a thorough checking over of the HPLC and careful adherence to the test procedures should be done to correct the discrepancy.
(vi) Modification of procedures for potential problems—Decomposition of the test compound. If the test compound decomposes in one or more of the aqueous solvents required during the period of the test at a rate such that an accurate value for water solubility cannot be obtained, then it will be necessary to carry out detailed transformation studies, such as hydrolysis studies. If decomposition is due to aqueous photolysis, then it will be necessary to carry out the studies in the dark, under red or yellow lights, or by any other suitable method to eliminate this transformation process.
(d) Data and reporting— (1) Test report. (i) For the test solution, report the weights to the nearest 0.1 mg of the test substance and n-octanol. Also report the weight percent and molar concentration of the test substance in the n-octanol; the density of n-octanol at 25 °C is 0.827 grams per milliliter (gm)/mL.
(ii) For each run provide the molar concentration of the test substance in water for each of three determinations, the mean value, and the standard deviation.
(iii) For each of the three determinations calculate the Kow as the ratio of the molar concentration of the test substance in n-octanol to the molar concentration in water. Also calculate and report the mean Kow and its standard deviation. Values of Kow shall be reported as their logarithms (log10 Kow).
(iv) Report the temperature (± 0.05 °C) at which the generator column was controlled during the test.
(v) For each reference compound report the individual values of log10 Kow and the average of the two runs.
(vi) For compounds that decompose at a rate such that a precise value for the solubility cannot be obtained, provide a statement to that effect.
(2) Specific analytical, calibration, and recovery procedures. (i) For the HPLC method describe and/or report:
(A) The method used to determine the sample-loop volume and the average and standard deviation of that volume.
(B) The average and standard deviation of the RF.
(C) The extraction solvent and the extraction efficiency used.
(D) Any changes made or problems encountered in the test procedures.
(ii) For the GC method report:
(A) The column and GC operating conditions of temperature and flow rate.
(B) The average and standard deviation of the average area per microliter obtained for each of the standard solutions.
(C) The form of the regression equation obtained in the calibration procedure. Start Printed Page 78760
(D) The extracting solvent and extraction efficiency used.
(E) The average and standard deviation of solute concentration in each collection vessel.
(F) Any changes made or problems encountered in the test procedure.
(iii) If another approved analytical method is used to determine the concentration of the test chemical in water, then all the important test conditions shall be reported.
(iv) If the concentration of the test substance in n-octanol is determined by an independent analytical method such as GC, provide a complete description of the method.
(e) References. For additional background information on this test guideline, the following references should be consulted. These references are available from the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., SW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, excluding legal holidays.
(1) Banerjee, S. et al., Water solubility and octanol/water partition coefficient of organics. Limitation of the solubility-partition coefficient correlation. Environmental Science and Technology 14:1227-1229 (1980).
(2) Bruggemann W.A. et al., Reversed-phase thin-layer chromatography of polynuclear aromatic hydrocarbons and chlorinated biphenyls. Relationship with hydrophobicity as measured by aqueous solubility and octanol/water partition coefficient. Journal of Chromatography 238: 335-346 (1982).
(3) Chiou, C.T. et al. Partition coefficient and bioaccumulation of selected organic chemicals. Environmental Science and Technology 11:475-478 (1977).
(4) Chiou, C.T. and Schmedding, D.W., Partitioning of organic compounds in octanol/water systems. Environmental Science and Technology 16:4-10 (1982).
(5) Chiou, C.T et al., Partition equilibria of nonionic organic compounds between soil, organic matter, and water. Environmental Science and Technology 17:227-231 (1983).
(6) DeVoe, H. et al. “Generator Columns and High Pressure Liquid Chromatography for Determining Aqueous Solubilities and Octanol-Water Partition Coefficients of Hydrophobic Substances,” Journal of Research of the National Bureau of Standards, 86:361-366 (1981).
(7) Fujita, T. et al. “A New Substituent Constant, Derived from Partition Coefficients.” Journal of the American Chemical Society, 86:5175 (1964).
(8) Hansch, C. and Leo, A. 1985 MEDCHEM Project, version 26. Pomona College, Claremont, CA. USA.
(9) Hansch, C. and Leo, A. Medchem Software Manual. CLOGP3 Users Guide. Release 3.32. December 1984. Medicinal Chemistry Project, Pomona College, Claremont, CA.
(10) Hawker, D.W. and Connell, D.W. Octanol-water partition coefficients of polychlorinated biphenyl congeners. Environmental Science and Technology 22:382-387 (1988).
(11) May, W.E. et al. “Determination of the aqueous solubility of polynuclear aromatic hydrocarbons by a coupled column liquid chromatographic technique,” Analytical Chemistry, 50:175-179 (1978).
(12) May, W.E. et al. “Determination of the Solubility Behavior of Some Polycyclic Aromatic Hydrocarbons in Water,” Analytical Chemistry 50:997-1000 (1978).
(13) Miller, M.M. et al. Aqueous solubilities, octanol/water partition coefficients and entropies of melting of chlorinated benzenes and biphenyls. Journal of Chemical and Engineering Data 29:184-190 (1984).
(14) Neely, W.B. et al. Partition Coefficient to Measure Bioconcentration Potential of Organic Chemicals in Fish, Environmental Science Technology, 8:113-115 (1974).
(15) Rappaport, R.A. and Eisenrich, S.J. Chromatographic determination of octanol-water partition coefficients (Kow's) for 58 polychlorinated biphenyl congeners. Environmental Science and Technology 18:163-170 (1984).
(16) Tewari, Y.B. et al. Aqueous solubility and octanol/water partition coefficients of organic compounds at 25 °C. Journal of Chemical and Engineering Data 27:451-454 (1982).
(17) Tulp, M.T.M. and Hutzinger, O. Some thoughts on aqueous solubilities and partition coefficients of PCB, and the mathematical correlation between bioaccumulation and physio-chemical properties. Chemosphere 10:849-860 (1978).
(18) Veith, G.D. et al. A rapid method for estimating log10 P for organic chemicals, Water Research 13:43-47 (1979).
(19) Wasik, S.P. et al. Octanol/water partition coefficient and aqueous solubilities of organic compounds, Report NBSIR 81-2406 (1981). National Bureau of Standards, U.S. Department of Commerce, Washington, DC.
(20) Woodburn, K.B. Measurement and application of the octanol/water partition coefficients for selected polychlorinated biphenyls. Master's Thesis (1982), University of Wisconsin at Madison, Madison, WI.
(21) Woodburn, K.B. et al. Generator column determination of octanol/water partition coefficients for selected polychlorinated biphenyl congeners. Environmental Science and Technology 18:457-459 (1984).
(22) ASTM D 1193-91 (Approved Sep 15, 1991), “Standard Specification for Reagent Water.” American Society for Testing and Materials (ASTM), 1916 Race St., Philadelphia, PA 19103.
TSCA water solubility: Column elution method; shake flask method.(a) Scope—(1) Applicability. This section is intended to meet the testing requirements of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Source. The source material used in developing this TSCA test guideline is the Office of Pollution Prevention, Pesticides and Toxics (OPPTS) harmonized test guideline 830.7840 (March 1998, revised final guideline). This source is available at the address in paragraph (f) of this section.
(b) Introductory information—(1) Prerequisites. Suitable analytical method, structural formula, vapor pressure curve, dissociation constant, and hydrolysis independence of pH (preliminary test).
(2) Coefficient of variation. The coefficient of variation on the mean values reported by the participants of the Organization for Economic Cooperation and Development (OECD) Laboratory Intercomparison Testing, Part I, 1979, appeared to be dependent on the chemicals tested and the test temperatures; it ranges from 0.05 to 0.34 for the column elution method, and from 0.03 to 1.12 for the flask method.
(3) Qualifying statements. (i) The method is not applicable to volatile substances. Care should be taken that the substances examined are as pure as possible and stable in water. It must be ascertained that the identity of the substance is not changed during the procedure.
(ii) The column elution method is not suitable for volatile substances. The carrier material used here may not yet be optimal. This method is intended for material with solubilities below approximately 10-2 gram/Liter (g/L).
(iii) The flask method is intended for materials with solubility above 10-2 g/L. It is not applicable to volatile substances; this method may pose difficulties in the case of surface-active materials.
(c) Method—(1) Introduction, purpose, scope, relevance, application, and limits of test. (i) A solution is a homogeneous mixture of different Start Printed Page 78761substances in a solvent. The particle sizes of the dispersed substances are of the same magnitude as molecules and ions; therefore, the smallest volumes which can be obtained from a solution are always of uniform composition.
(ii) Solubility in water is a significant parameter because:
(A) The spatial and temporal movement (mobility) of a substance is largely determined by its solubility in water.
(B) Water soluble substances gain ready access to humans and other living organisms.
(C) The knowledge of the solubility in water is a prerequisite for testing biological degradation and bioaccumulation in water and for other tests.
(iii) No single method is available to cover the whole range of solubilities in water, from relatively soluble to very low-soluble chemicals. A general test guideline for the determination of the solubility in water must include methods which cover the whole range of water soluble substances. Therefore, this section includes two methods:
(A) One which applies to substances with low solubilities (<10-2 g/L), referred to as the “column elution method.”
(B) The other which applies to substances with higher solubilities (≤10-2 g/L), referred to as the “flask method.”
(2) Definition. The solubility in water of a substance is specified by the saturation mass concentration of the substance in water and is a function of temperature. The solubility in water is specified in units of weight per volume of solution. The SI-unit is killogram/meter (kg/m)3; g/L may also be used.
(3) Reference substances. The reference substances need not be employed in all cases when investigating a new substance. They are provided primarily so that calibration of the method may be performed from time to time and to offer the chance to compare the results when another method is applied. The values presented in table 1 of this section are not necessarily representative of the results which can be obtained with this test method as they have been derived from an earlier version of the test method.
Table 1.—Data for Reference Substances
Method T, °C Mean (milligram (mg)/L) Range (mg/L) No. of labs Fluoranthene Elution method 15 0.275 0.104 to 0.920 6 25 0.373 0.198 to 1.050 7 Hexachlorobenzene Elution method 15 9.21 × 10-3 2.06 × 10-3 to 2.16 × 10-2 6 25 9.96 × 10-3 1.19 × 10-3 to 2.31 × 10-2 7 g-Hexachlorocyclohexane Elution method 15 6.50 4.43 to 10.5 6 25 9.20 6.64 to 14.5 7 2,4-Dichlorophenoxyacetic acid Flask method 15 0.633 0.380 to 0.764 5 25 0.812 0.655 to 0.927 5 Mercury(II) chloride: Flask method 15 53.0 47.7 to 56.5 4 25 66.4 58.3 to 70.4 4 4-Nitrophenol: Flask method 15 9.95 8.88 to 10.9 6 25 14.8 13.8 to 15.9 6 (4) Principle of the test methods. The approximate amount of the sample and the time necessary to achieve the saturation mass concentration should be determined in a simple preliminary test.
(i) Column elution method. This method is based on the elution of a test substance with water from a microcolumn which is charged with an inert carrier material such as glass beads, silica gel, or sand, and an excess of test substance. The water solubility is determined when the mass concentration of the eluate is constant. This is shown by a concentration plateau as a function of time in the following figure 1: Start Printed Page 78762
Figure 1.—Concentration versus Time of Substance in the Eluate
(ii) Flask method. In this method, the substance (solids must be pulverized) is dissolved in water at a temperature somewhat above the test temperature. When saturation is achieved, the mixture is cooled and kept at the test temperature, stirring as long as necessary to reach equilibrium. Such a procedure is described in the reference listed in paragraph (f)(2) of this section. Subsequently, the mass concentration of the substance in the aqueous solution, which must not contain any undissolved particles, is determined by a suitable analytical method.
(5) Quality criteria—(i) Repeatability. For the column elution method <30% is acceptable; for the flask method <15% should be observed.
(ii) Sensitivity. This depends upon the method of analysis, but mass concentration determinations down to at least 10-6 g/L can be determined.
(iii) Specificity. These methods should only be applied to:
(A) Pure substance.
(B) Substances that are stable in water.
(C) Slightly soluble substances, i.e. <10-2 g/L for the column elution method.
(D) Organic substances for the column elution method.
(iv) Possibility of standardization. These methods can be standardized.
(d) Description of the test procedures—(1) Preparations—(i) Apparatus—(A) Column elution method. (1) The schematic arrangement of the system is presented in the following figure 2:
Figure 2.—Schematic Test Arrangement
(2) Although any size is acceptable, provided it meets the criteria for reproducibility and sensitivity. The column should provide for a head space of at least five bed-volumes of water and a minimum of five samples. Alternatively, the size can be reduced if make-up solvent is employed to replace the initial five bed-volumes removed with impurities. A suitable microcolumn is shown in the following figure 3: Start Printed Page 78763
Figure 3.—Microcolumn (all dimensions in millimeters)
(3) The column should be connected to a recycling pump capable of controlling flows of approximately 25 mL/hours (h). The pump is connected with polytetrafluoroethylene and/or glass connections. The column and pump, when assembled, should have provision for sampling the effluent and equilibrating the head space at atmospheric pressure. The column material is supported with a small (5 millimeter (mm)) plug of glass wool, which must also serve to filter particles.
(B) Flask method. For the flask method, the following material is needed:
(1) Normal laboratory glassware and instrumentation.
(2) A device suitable for the agitation of solutions under controlled constant temperatures.
(3) A centrifuge (preferably thermostatted), if required with emulsions.
(4) Equipment for analytical determinations.
(2) Reagents. The substance to be tested should be as pure as possible, particularly in the flask method where purification is not provided. The carrier material for the column elution method should be inert. Possible materials which can be employed are glass beads and silica. A suitable volatile solvent of analytical reaction quality should be used to apply the test substance to the carrier material. Double distilled water from glass or quartz apparatus should be employed as the eluent or solvent. Water directly from an ion exchanger must not be used.
(3) Test conditions. The test is preferably run at 20 ± 0.5 °C (293 °K). If temperature dependence is suspected in the solubility (≤ 3%/°C), two other temperatures should also be used—both differing from each other and the initially chosen temperature by 10 °C. In this case the temperature control should be ± 0.1 °C. One of these additional temperatures should be below the initial temperature. The chosen temperature(s) should be kept constant in all parts of the equipment (including the leveling vessel).
(4) Performance of the tests—(i) Preliminary test. (A) To approximately 0.1 g of the sample (solid substances must be pulverized) in a glass-stoppered 10 milliliter (mL) graduated cylinder, increasing volumes of distilled water at room temperature are added according to the steps shown in Table 2 of this section:
Start Printed Page 78764Table 2.—Determination of Solubility
Solubility data step 1 step 2 step 3 step 4 step 5 step 6 step 7 Total volume H2 O added (mL) 0.1 0.5 1 2 10 100 ≤100 Approximate solubility (g/L) ≤1,000 200 100 50 10 1 <1 (B) After each addition of water to give the indicated total volume, the mixture is shaken vigorously for 10 min and is visually checked for any undissolved parts of the sample. If, after a total of 10 mL of water has been added (step 5), the sample or parts of it remain undissolved, the contents of the measuring cylinder is transferred to a 100 mL measuring cylinder which is then filled up with water to 100 mL (step 6) and shaken. At lower solubilities the time required to dissolve a substance can be considerably long (24 h should be allowed). The approximate solubility is given in the table under that volume of added water in which complete dissolution of the sample occurs. If the substance is still apparently insoluble, further dilution should be undertaken to ascertain whether the column elution or flask solubility method should be used.
(ii) Column elution—(A) Apparatus. (1) The equipment is arranged as shown in figures 2 and 3 in paragraphs (d)(1)(i)(A)(1) and (d)(1)(i)(A)(2) of this section. Approximately 600 milligrams (mg) of carrier material is weighed and transferred to a 50 mL round-bottom flask. A suitable, weighed amount of test substance is dissolved in the chosen solvent, and an appropriate amount of the test substance solution is added to the carrier material. The solvent must be completely evaporated, e.g. in a rotary evaporator; otherwise water saturation of the carrier is not achieved due to partition effects on the surface of the carrier.
(2) The loading of carrier material may cause problems (erroneous results) if the test substance is deposited as an oil or a different crystal phase. The problem should be examined experimentally.
(3) The loaded carrier material is allowed to soak for about 2 h in approximately 5 mL of water, and then the suspension is added to the microcolumn. Alternatively, dry loaded carrier material may be poured in the microcolumn, which has been filled with water and then equilibrated for approximately 2 h.
(B) Test procedure. The elution of the substance from the carrier material can be carried out in two different ways: Leveling vessel or circulating pump. The two principles should be used alternatively.
(1) Leveling vessel, see figure 3 in paragraph (d)(1)(i)(A)(2) and figure 4 in paragraph (d)(4)(iii) of this section.
(i) The connection to the leveling vessel is made by using a ground glass joint which is connected by teflon tubing. It is recommended that a flow rate of approximately 25 mL/h be used. Successive eluate fractions should be collected and analyzed by the chosen method.
(ii) Fractions from the middle eluate range where the concentrations are constant (± 30%) in at least five consecutive fractions are used to determine the solubility in water.
(iii) A second run is to be performed at half the flow rate of the first. If the results of the two runs are in agreement, the test is satisfactory; if there is a higher apparent solubility with the lower flow rate, then the halving of the flow rate must continue until two successive runs give the same solubility.
(2) Circulating pump, see figures 2 and 3 in paragraphs (d)(1)(i)(A)(1) and (d)(1)(i)(A)(2) of this section.
(i) With this apparatus, the microcolumn must be modified. A stopcock with 2-way action must be used, see figure 3 in paragraph (d)(1)(i)(A)(2) of this section). The circulating pump can be, e.g. a peristaltic pump (be careful that no contamination and/or adsorption occurs with the tube material) or a membrane pump.
(ii) The flow through the column is started. It is recommended that a flow rate of approximately 25 mL/h be used (approximately 10 bed volumes per h for the described column). The first five-bed volumes (minimum) are discarded to remove water soluble impurities.
(iii) Following this, the recycling pump is connected and the apparatus allowed to run until equilibration is established, as defined by five successive samples whose concentrations do not differ by more than 30% in a random fashion (see paragraph (f)(2) of this section). These samples should be separated from each other by time intervals corresponding to the passage of at least 10 bed-volumes of the eluent.
(3) In both cases (using a circulation pump or a leveling vessel) the fractions should be checked for the presence of colloidal matter by examination for the Tyndall effect (light scattering). Presence of such particles invalidates the results, and the test should be repeated with improvements in the filtering action of the column. The pH of each sample should be recorded. A second run should be performed at the same temperature.
(iii) Flask method: Test procedure. The quantity of material necessary to saturate the desired volume of water is estimated from the preliminary test. The volume of water required will depend on the analytical method and the solubility range. About five times the quantity of material determined in paragraph (d)(4)(i)(A) of this section is weighed into each of three glass vessels fitted with glass stoppers (e.g. centrifuge tubes, flasks). The chosen volume of water is added to each vessel, and the vessels are tightly stoppered. The closed vessels are then agitated at 30 °C. (A shaking or stirring device capable of operating at constant temperature should be used, e.g. magnetic stirring in a thermostatically controlled water bath.) After 1 day, one of the vessels is removed and re-equilibrated for 24 h at the test temperature with occasional shaking. The contents of the vessel are then centrifuged at the test temperature, and the concentration of compound in the clear aqueous phase is determined by a suitable analytical method. The other two flasks are treated similarly after initial equilibration at 30 °C for 2 and 3 days, respectively. If the concentration results from at least the last two vessels agree with the required reproducibility, the test is satisfactory. The whole test should be repeated, using longer equilibration times if the results from vessels one, two, and three show a tendency to increasing values. The arrangement of the apparatus is shown in the following figure 4: Start Printed Page 78765
Figure 4.—Test Arrangement for the Determination of Solubility in Water of Slightly Soluble, Low Volatility Organic Substances
1 = Leveling vessel (e.g. 2.5 L chemical flask)
2 = Column (see figure 3 in paragraph (d)(1)(i)(A)(2) of this section)
3 = Fraction accumulator
4 = Thermostat
5 = Teflon tubing
6 = Glass stopper
7 = Water line (between thermostat and column, inner diameter: approximately 8 mm)
(iv) Analysis. A substance-specific analytical method is required for these determinations, since small amounts of soluble impurities can cause large errors in the measured solubility. Examples of such methods are gas or liquid chromatography, titration methods, photometric methods, and polarographic methods.
(e) Data and reporting—(1) Column elution method—(i) Treatment of results. The mean value from at least five consecutive samples taken from the saturation plateau (figure 1 in paragraph (c)(4)(i) of this section) should be determined for each run, as should the standard deviation. A comparison should be made between the two means to ensure that they agree with a repeatability of less than 30%.
(ii) Test report. The report should contain an indication of the results of the preliminary test plus the following information:
(A) The individual concentrations, flow rates and pHs of each samples.
(B) The means and standard deviations from at least five samples from the saturation plateau of each run.
(C) The average of the two successive, acceptable runs.
(D) The temperature of the runs.
(E) The method of analysis employed.
(F) The nature of the carrier material employed.
(G) Loading of carrier material.
(H) Solvent used.
(I) Statement that the identity of the substance in the saturated solution has been proved.
(2) Flask method—(i) Treatment of results. The individual results should be given for each of the three flasks and those results deemed to be constant (repeatability <15%) should be averaged and given in units of mass per volume of solution. This may require the conversion of mass units to volume units, using the density when the solubility is very high (100 g/L).
(ii) Test report. The report should include the following information:
(A) The individual analytical determinations and the average where more than one value was determined for each flask.
(B) The average of the value for the different flasks which were in agreement.
(C) The test temperature.
(D) The analytical method employed.
(f) References. For additional information on this test guideline, the following references should be consulted. These references are available from the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., SW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, excluding legal holidays.
(1) Veith, G.D. and V.M. Comstock. Apparatus for continuously saturating water with hydrophobic organic chemicals. Journal of the Fishing Research Board of Canada 32:1849-1851 (1975).
(2) Organization for Economic Cooperation and Development, Guidelines for The Testing of Chemicals, OECD 105, Water Solubility (Column Elution Method—Shake Flask Method), OECD, Paris, France (1981).
TSCA water solubility: Generator column method.(a) Scope—(1) Applicability. This section is intended to meet the testing requirements of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Source. The source material used in developing this TSCA test guideline is the Office of Pollution Prevention, Pesticides and Toxics (OPPTS) harmonized test guideline 830.7860 (March 1998, revised final guideline). The source is available at the address in paragraph (e) of this section.
(b) Introduction— (1) Purpose. (i) The water solubility of a chemical is defined as the equilibrium concentration of the chemical in a saturated aqueous solution at a given temperature and pressure. The aqueous phase solubility is an important factor in governing the movement, distribution, and rate of degradation of chemicals in the environment. Substances that are relatively water soluble are more likely to be widely distributed by the hydrologic cycle than those which are Start Printed Page 78766relatively insoluble. Furthermore, substances with higher water solubility are more likely to undergo microbial or chemical degradation in the environment because dissolution makes them “available” to interact and, therefore, react with other chemicals and microorganisms. Both the extent and rate of degradation via hydrolysis, photolysis, oxidation, reduction, and biodegradation depend on a chemical being soluble in water (i.e., homogeneous kinetics).
(ii) Water provides the medium in which many organisms live, and water is a major component of the internal environment of all living organisms (except for dormant stages of certain life forms). Even organisms which are adapted to life in a gaseous environment require water for normal functioning. Water is thus the medium through which most other chemicals are transported to and into living cells. As a result, the extent to which chemicals dissolve in water will be a major determinant for movement through the environment and entry into living systems.
(iii) The water solubility of a chemical also has an effect on its sorption into and desorption from soils and sediments, and on volatilization from aqueous media. The more soluble a chemical substance is, the less likely it is to sorb to soils and sediments and the less likely it is to volatilize from water. Finally, the design of most chemical tests and many ecological and health tests requires precise knowledge of the water solubility of the chemical to be tested.
(2) Definitions. The following definitions apply to this section.
Concentration (C) of a solution is the amount of solute in a given amount of solvent or solution and can be expressed as a weight/weight or weight/volume relationship. The conversion from a weight relationship to one of volume incorporates density as a factor. For dilute aqueous solutions, the density of the solvent is approximately equal to the density of the solution; thus, concentrations expressed in milligrams per liter (mg/L) are approximately equal to 10-3 g/103 g or parts per million (ppm); those expressed in micrograms per liter (mg/L) are approximately equal to 10-6 g/103 g or parts per billion (ppb). In addition, concentration can be expressed in terms of molarity, normality, molality, and mole fraction. For example, to convert from weight/volume to molarity molecular mass is incorporated as a factor.
Density is the mass of a unit volume of a material. It is a function of temperature, hence the temperature at which it is measured should be specified. For a solid, it is the density of the impermeable portion rather than the bulk density. For solids and liquids, suitable units of measurement are grams per cubic centimeter (g/cm3). The density of a solution is the mass of a unit volume of the solution and suitable units of measurement are g/cm3.
Extractor column is used to extract the solute from the saturated solutions produced by the generator column. After extraction onto a chromatographic support, the solute is eluted with a solvent/water mixture and subsequently analyzed by high-pressure liquid chromatography (HPLC), gas chromatography (GC), or any other suitable analytical procedure. A detailed description of the preparation of the extractor column is given in paragraph (c)(1)(i)(D) of this section.
Generator column is used to produce or generate saturated solutions of a solute in a solvent. The column, see figure 1 in paragraph (c)(1)(i)(A) of this section, is packed with a solid support coated with the solute, i.e., the organic compound whose solubility is to be determined. When water (the solvent) is pumped through the column, saturated solutions of the solute are generated. Preparation of the generator column is described in paragraph (c)(1)(i)(A) of this section.
Response factor (RF) is the solute concentration required to give a 1 unit area chromatographic peak or 1 unit output from the HPLC recording integrator at a particular recorder attenuation. The factor is required to convert from units of area to units of concentration. The determination of the RF is given in paragraph (c)(3)(ii)(B)(2) of this section.
Sample loop is a 1/16 inch (in) outer diameter (O.D.) (1.6 millimeter (mm)) stainless steel tube with an internal volume between 20 and 50 mL. The loop is attached to the sample injection valve of the HPLC and is used to inject standard solutions into the mobile phase of the HPLC when determining the RF for the recording integrator. The exact volume of the loop must be determined as described in paragraph (c)(3)(ii)(B)(1) of this section when the HPLC method is used.
Saturated solution is a solution in which the dissolved solute is in equilibrium with an excess of undissolved solute; or a solution in equilibrium such that at a fixed temperature and pressure, the concentration of the solute in the solution is at its maximum value and will not change even in the presence of an excess of solute.
Solution is a homogeneous mixture of two or more substances constituting a single phase.
(3) Principle of the test method. (i) This test method is based on the dynamic coupled column liquid chromatographic (DCCLC) technique for determining the aqueous solubility of organic compounds that was initially developed by May et al. (as described in the references listed in paragraphs (e)(5) and (e)(6) of this section), modified by DeVoe et al. (as described in the reference listed in paragraph (e)(1) of this section), and finalized by Wasik et al. (as described in the reference listed in paragraph (e)(11) of this section). The DCCLC technique utilizes a generator column, extractor column and HPLC coupled or interconnected to provide a continuous closed flow system. Saturated aqueous solutions of the test compound are produced by pumping water through the generator column that is packed with a solid support coated with the compound. The compound is extracted from the saturated solution onto an extractor column, then eluted from the extractor column with a solvent/water mixture and subsequently analyzed by HPLC using a variable wavelength ultraviolet (UV) detector operating at a suitable wavelength. Chromatogram peaks are recorded and integrated using a recording integrator. The concentration of the compound in the effluent from the generator column, i.e., the water solubility of the compound, is determined from the mass of the compound (solute) extracted from a measured volume of water (solvent).
(ii) Since the HPLC method is only applicable to compounds that absorb in the UV, an alternate GC method, or any other reliable procedure (which must be approved by OPPTS), can be used for those compounds that do not absorb in the UV. In the GC method the saturated solutions produced in the generator column are extracted using an appropriate organic solvent that is subsequently injected into the GC, or any other suitable analytical device, for analysis of the test compound.
(4) Reference chemicals. Table 1 of this section lists the water solubilities at 25 °C for a number of reference chemicals as obtained from the scientific literature. The data from Wasik et al. (as described in the reference listed in paragraph (e)(11) of this section), Miller et al. and Tewari et al. (as described in the references listed in paragraphs (e)(7) and (e)(10) of this section, respectively) were obtained from the generator column method. The water solubilities data were also obtained from Mackay et al. and Yalkowski et al. (as described in the Start Printed Page 78767references listed in paragraphs (e)(4) and (e)(12) of this section, respectively) and other scientists by the conventional shake flask method. These data have been provided primarily so that the generator column method can be calibrated from time to time and to allow the chemical testing laboratory an opportunity to compare its results with those listed in table 1 of this section. The water solubility values at 25 °C reported by Yalkowski et al. are their preferred values and, in general, represent the best available water solubility data at 25 °C. The testing laboratory has the option of choosing its own reference chemicals, but references must be given to establish the validity of the measured values of the water solubility.
Table 1.—Water Solubilities at 25 °C of Some Reference Chemicals
Reference chemical Water solubility (ppm at 25 °C) Wasik (generator column method) Yalkowski15 Other literature references 2-Heptanone 2 4080 4300 5 4330 1-Chlorobutane 2 873 872.9 7 666 Ethylbenzene 2 187 208 7 162 1,2,3-Trimethylbenzene 2 65.5 75.2 7 48.2 Biphenyl 310 6.71 7.48 8 6.62 Phenanthrene 4 1.002 1.212 — 2,4,6-Trichlorobiphenyl 310 0.226 0.225 8 0.119 2,3,4,5-Tetrachlorobiphenyl 310 0.0209 0.01396 8 0.0192 Hexachlorobenzene — 0.004669 9 0.00996 2,3,4,5,6-Pentachlorobiphenyl 310 0.00548 0.004016 8 0.0068 1 Preferred water solubility at 25 °C by Yalkowski et al. (1990) in paragraph (e)(12) of this section based on a critical review of all the experimental water solubility data published. 2 Tewari et al. (1982) in paragraph (e)(10) of this section. 3 Leifer et al. (1983) in paragraph (e)(3) of this section. 4 May, Wasik, and Freeman (1978, 1978a) in paragraphs (e)(5) and (6) of this section. 5 Yalkowski et al. (1990) in paragraph (e)(12) of this section. 6 Hansch et al. (1968) in paragraph (e)(2) of this section. 7 Sutton and Calder (1975) in paragraph (e)(9) of this section. 8 Mackay et al. (1980) in paragraph (e)(4) of this section. 9 The elution chromatographic method from Organization for Economic Cooperation and Development (OECD) (1981) in paragraph (e)(8) of this section. 10 Miller et al. (1984) in paragraph (e)(7) of this section. (5) Applicability and specificity. (i) Procedures are described in this section to determine the water solubility for liquid or solid compounds. The water solubility can be determined in very pure water, buffer solution for compounds that reversibly ionize or protonate, or in artificial seawater as a function of temperature (i.e., in the range of temperatures of environmental concern). This section is not applicable to the water solubility of gases.
(ii) This section is designed to determine the water solubility of a solid or liquid test chemical in the range of 1 ppb to 5,000 ppm. For chemicals whose solubility is below 1 ppb, the water solubility should be characterized as “less than 1 ppb” with no further quantification. For solubilities greater than 5,000 ppm, the shake flask method should be used, see paragraph (e)(15) of this section.
(c) Test procedure— (1) Test conditions— (i) Special laboratory equipment—(A) Generator column. (1) Either of two different designs shall be used depending on whether the eluted aqueous phase is analyzed by HPLC in paragraph (c)(3)(ii) of this section or by solvent extraction followed by GC (or any other reliable quantitative) analysis of solvent extract in paragraph (c)(3)(iv) of this section. The design of the generator column is shown in the following figure 1:
Figure 1—Generator Column
(2) The column consists of a 6 mm (1/4 in) O.D. pyrex tube joined to a short enlarged section of 9 mm pyrex tubing which in turn is connected to another section of 6 mm (1/4 in) O.D. pyrex tubing. Connections to the inlet teflon tubing (1/8 in O.D.) and to the outlet stainless steel tubing (1/16 in O.D.) shall be made by means of stainless steel fittings with teflon ferrules. The column is enclosed in a water jacket for temperature control as shown in the following figure 2: Start Printed Page 78768
Figure 2—Setup Showing Generator Column Enclosed in a Water Jacket and Overall Arrangement of the Apparatus Used in the GC Method
(B) Constant temperature bath with circulation pump-bath and capable of controlling temperature to ± 0.05 °C, see paragraph (c)(3) of this section.
(C) HPLC equipped with a variable wavelenth UV absorption detector operating at a suitable wavelength and a recording integrator in paragraph (c)(3)(ii) of this section.
(D) Extractor column—6.6 × 0.6 cm stainless steel tube with end fittings containing 5 mm frits filled with a superficially porous phase packing (Bondapack C18/Corasil: Waters Associates) in paragraph (c)(3)(ii) of this section.
(E) Two 6-port high-pressure rotary switching valves in paragraph (c)(3)(ii) of this section.
(F) Collection vessel—8 × 3/4 in section of pyrex tubing with a flat bottom connected to a short section of 3/8 in O.D. borosilicate glass tubing in figure 2 in paragraph (c)(1)(i)(A)(2) of this section. The collecting vessel is sealed with a 3/8 in teflon cap fitting in paragraph (c)(3)(iii) of this section.
(G) GC, or any other reliable analytical equipment, which has a detector sensitive to the solute of interest in paragraph (c)(3)(iii) of this section.
(ii) Purity of water. Water meeting appropriate American Society for Testing and Materials (ASTM) Type II standards, or an equivalent grade, are recommended to minimize the effects of dissolved salts and other impurities on water solubility. ASTM Type II water is presented in the reference listed in paragraph (e)(13) of this section.
(iii) Purity of solvents. All solvents used in this method must be reagent or HPLC grade. Solvents must contain no impurities which could interfere with the determination of the test compound.
(iv) Seawater. When the water solubility in seawater is desired, the artificial seawater described in paragraph (c)(2)(ii) of this section must be used.
(v) Effect of pH on solubility. For chemicals that reversibly ionize or protonate with a pKa or pKb between 3 and 11, experiments must be performed at pH's 5.0, 7.0, and 9.0 using appropriate buffers.
(2) Preparation of reagents and solutions— (i) Buffer solutions. Prepare buffer solutions as follows:
(A) pH 3.0—to 250 mL of 0.10M potassium hydrogen phosphate add 111 mL of 0.10 M hydrochloric acid; adjust the final volume to 500 mL with reagent grade water.
(B) pH 5.0—to 250 mL of 0.1M potassium hydrogen phthalate add 113 mL of 0.1M sodium hydroxide; adjust the final volume to 500 mL with reagent grade water.
(C) pH 7.0—to 250 mL of 0.1M potassium dihydrogen phosphate add 145 mL of 0.1M sodium hydroxide; adjust the final volume to 500 mL with reagent grade water.
(D) pH 9.0—to 250 mL of 0.075M borax add 69 mL of 0.1M HCl; adjust the final volume to 500 mL with reagent grade water.
(E) pH 11.0—to 250 mL of 0.05 M sodium bicarbonate add 3 mL of 0.10 M sodium hydroxide; adjust the final volume to 500 mL with reagent grade water.
(ii) Check the pH of each buffer solution with a pH meter at 25 °C and adjust to pH 5.0, 7.0, or 9.0, if necessary. If the pH of the solution has changed by ±0.2 pH units or more after the addition of the test compound, then a more concentrated buffer is required for that pH determination. The sponsor should then choose a more suitable buffer.
(iii) Artificial seawater. Add the reagent-grade chemicals listed in table 2 of this section in the specified amounts and order to 890 mL of reagent-grade water. Each chemical shall be dissolved before another one is added.
Table 2.—Constituents of Artificial Seawater1
Chemical Amount NaF 3 mg SrCl2.6H2 O 20 mg H3 BO3 30 mg KBr 100 mg KCl 700 mg CaCl2.2H2 O 1.47 gram (g) Na2 SO4 4.00 g MgCl2.6H2 O 10.78 g NaCl 23.50 g Na2 SiO3.9H2 O 20 mg NaHCO3 200 mg 1 If the resulting solution is diluted to 1 L, the salinity should be 34±0.5 g/kilogram (kg) and the pH 8.0±0.2. The desired test salinity is attained by dilution at time of use. (3) Performance of the test. Using either the procedures in paragraph (c)(3)(ii) or (c)(3)(iii) of this section, determine the water solubility of the test compound at 25 °C in reagent-grade water or buffer solution, as appropriate. Under certain circumstances, it may be necessary to determine the water solubility of a test compound at 25 °C in artificial seawater. The water solubility can also be determined at other temperatures of environmental concern by adjusting the temperature of the water bath to the appropriate temperature.
(i) Prior to the determination of the water solubility of the test chemical, two procedures shall be followed.
(A) The saturated aqueous solution leaving the generator column must be tested for the presence of an emulsion, using a Tyndall procedure. If colloids are present, they must be eliminated prior to the injection into the extractor column. This may be achieved by lowering the flow rate of the water.
(B) The efficiency of the removal of the solute (i.e. test chemical) by the solvent extraction from the extraction column must be determined and used in the determination of the water solubility of the test chemical.
(ii) Procedure A—HPLC method— (A) Scope. (1) Procedure A covers the determination of the aqueous solubility of compounds which absorb in the UV.
(i) The HPLC analytical system is shown schematically in the following figure 3: Start Printed Page 78769
Figure 3—Schematic of HPLC—Generator Column Flow System
(ii) Two reciprocating piston pumps deliver the mobile phase (water or solvent/water mixture) through two 6-port high-pressure rotary valves and a 30 × 0.6 cm C18/Corasil analytical column to a variable wavelength UV absorption detector operating at a suitable wavelength; chromatogram peaks are recorded and integrated with a recording integrator. One of the 6-port valves is the sample injection valve used for injecting samples of standard solutions of the solute in an appropriate concentration for determining RFs of standard solutions of basic chromate for determining the sample-loop volume. The other 6-port valve in the system serves as a switching valve for the extractor column which is used to remove solute from the aqueous solutions.
(2) The general procedure for analyzing the aqueous phase is as follows (a detailed procedure is given in paragraph (c)(3)(ii)(B)(4) of this section).
(i) Direct the aqueous solution to “Waste,” see figure 3 in paragraph (c)(3)(ii)(A)(1)(i) of this section, with the switching valve in the inject position in order to equilibrate internal surfaces with the solution, thus ensuring that the analyzed sample would not be depleted by solute adsorption on surfaces upstream from the valve.
(ii) At the same time, water is pumped from the HPLC pumps in order to displace the solvent from the extractor column.
(iii) The switching valve is next changed to the load position to divert a sample of the solution through the extractor column, and the liquid leaving this column is collected in a weighing bottle. During this extraction step, the mobile phase is changed to a solvent/water mixture to condition the analytical column.
(iv) After the desired volume of sample is extracted, the switching valve is returned to the inject position for elution and analysis. Assuming that there is no breakthrough of solute from the extractor column during the extraction step, the chromatographic peak represents all of the solute in the sample, provided that the extraction efficiency is 100%. If the extraction efficiency is less than 100%, then the extraction efficiency shall be used to determine the actual weight of the solute extracted.
(v) The solute concentration in the aqueous phase is calculated from the peak area and the weight of the extracted liquid collected in the weighing bottle.
(B) Determinations—(1) Sample-loop volume. Accurate measurement of the sample loop may be accomplished by using the spectrophotometric method of Devoe et al. under paragraph (e)(1) of this section. For this method measure absorbance, Aloop, at 373 nm of at least three solutions, each of which is prepared by collecting from the sample valve an appropriate number, n, of loopfuls of an aqueous stock solution of K2 CrO4 (1.3% by weight) and diluting to 50 mL with 0.2% KOH. (For a 20 mL loop, use n = 5; for a 50 mL loop, use n = 2.) Also measure the absorbance, Astock, of the same stock solution after diluting 1:500 with 0.2% KOH. Calculate the loop volume to the nearest 0.1 mL using the equation:
Equation 1:
(2) RF. (i) For all determinations adjust the mobile phase solvent/water ratio and flow rate to obtain a reasonable retention time on the HPLC column. For example, typical concentrations of solvent in the mobile phase range from 50 to 100% while flow rates range from 1 to 3 mL/min; these conditions give a 3 to 5 min retention time.
(ii) Prepare standard solutions of known concentrations of the solute in a suitable solvent. Concentrations must give a recorder response within the maximum response of the detector. Inject samples of each standard solution into the HPLC system using the calibrated sample loop. Obtain an average peak area from at least three injections of each standard sample at a set absorbance unit full scale (AUFS), i.e., at the same absorbance scale attenuation setting.
(iii) Calculate the RF from the following equation:
Equation 2:
(3) Loading of the generator column. (i) The design of the generator column was described in paragraph (c)(1)(i) of this section and is shown in figure 1 in paragraph (c)(1)(i)(A) of this section. To pack the column, a plug of silanized glass wool is inserted into one end of the 6 mm pyrex tubing. Silanized diatomaceous silica support (about 0.5g 100-120 mesh Chromosorb (W) chromatographic support material) is poured into the tube with tapping and retained with a second plug of silanized glass wool.
(ii) If the solute is a liquid, the column is loaded by pulling the liquid solute through the dry support with gentle suction. If the solute is a solid, a 1% Start Printed Page 78770solution of the solid in a volatile solvent is added to the dry packing. The solvent is then distilled off the column under reduced pressure. After loading the column draw water up through the column to remove entrapped air.
(4) Analysis of the solute. Use the following procedure to collect and analyze the solute.
(i) With the switching valve (figure 3 in paragraph (c)(3)(ii)(A)(1)(i) of this section) in the inject position (i.e., water to waste), pump water through the generator column at a flow rate of approximately 1 mL/min for approximately 5 minutes (min) to bring the system into equilibrium. Pump water to the generator column by means of a minipump or pressurized water reservoir as shown in the following figure 4:
Figure 4—Water Reservoir for GC Method
(ii) Flush out the solvent that remains in the system from previous runs by changing the mobile phase to 100% H2 O and allowing the water to reach the HPLC detector, as indicated by a negative reading. As soon as this occurs, place a 25 mL weighing bottle (weighed to the nearest mg) at the waste position and immediately turn the switching valve to the load position.
(iii) Collect an amount of water (as determined by trial and error) in the weighing bottle, corresponding to the amount of solute adsorbed by the extractor column that gives a large on-scale detector response. During this extraction step, switch back to the original HPLC mobile phase composition, i.e., solvent/water mixture, to condition the HPLC analytical column.
(iv) After the desired volume of sample has been extracted, turn the switching valve back to the inject position (figure 3 in paragraph (c)(3)(ii)(A)(1)(i) of this section); at the same time turn on the recording integrator. The solvent/water mobile phase will elute the solute from the extractor column and transfer the solute to the HPLC analytical column.
(v) Remove the weighing bottle, cap it, and replace it with the waste container. Determine the weight of water collected to the nearest mg and record the corresponding peak area. Using the same AUFS setting repeat the analysis of the solute at least two more times and determine the average ratio of peak area to grams of water collected. In this equation, s = solubility (M), RF = response factor, Vloop = sample-loop volume (L), and R = ratio of area to grams of water. Calculate the solute solubility in water using the following equation:
Equation 3:
(iii) Procedure B—GC method— (A) Scope. In the GC method, or any other analytical method, aqueous solutions from the generator column enter a collecting vessel (figure 2 in paragraph (c)(1)(i)(A)(2) of this section) containing a known weight of extracting solvent which is immiscible in water. The outlet of the generator column is positioned such that the aqueous phase always enters below the extracting solvent. After the aqueous phase is collected, the collecting vessel is stoppered and the quantity of aqueous phase is determined by weighing. The solvent and the aqueous phase are equilibrated by slowly rotating the collecting vessel. The extraction efficiency of the solvent must be determined at this time. A small amount of the extracting solvent is removed and injected into a gas chromograph equipped with an appropriate detector. The solute concentration in the aqueous phase is determined from a calibration curve constructed using known concentrations of the solute.
(B) Alternative method. If another (approved) analytical method is used instead of the GC, that method shall be used to determine quantitatively the amount of solute present in the extraction solvent.
(C) Determinations—(1) Calibration curve. (i) Prepare solute standard solutions of concentrations covering the range of the solute solubility. Select a column and optimum GC operating conditions for resolution between the solute and solvent and the solute and extracting solvent. Inject a known volume of each standard solution into the injection port of the GC. For each standard solution determine the average of the ratio R of peak area to volume (in microliters) for three chromatographic peaks from three injections.
(ii) After running all the standard solutions, determine the coefficients, a and b, using a linear regression equation of C vs. R in the following form:
Equation 4:
(iii) If another analytical method is used, the procedures described in paragraph (c)(3)(iii)(C)(1) of this section shall be used to determine quantitatively the amount of solute in the extraction solvent.
(2) Loading of the generator column. The generator column is packed and loaded with solute in the same manner as for the HPLC method described under paragraph (c)(3)(ii)(B)(3) of this section. As shown in figure 2 in paragraph (c)(1)(i)(A)(2) of this section, attach approximately 20 cm of straight stainless steel tubing to the bottom of the generator column. Connect the top of the generator column to a water reservoir (figure 4 in paragraph (c)(3)(ii)(B)(4)(i) of this section) using teflon tubing. Use air or nitrogen pressure (5 PSI) from an air or nitrogen cylinder to force water from the reservoir through the column. Collect water in an Erlenmeyer flask for approximately 15 min while the solute concentration in water equilibrates; longer time may be required for less soluble compounds.
(3) Collection and extraction of the solute. During the equilibration time, add a known weight of extracting solvent to a collection vessel which can be capped. The extracting solvent should cover the bottom of the collection vessel to a depth sufficient to submerge the collecting tube but still maintain 100:1 water/solvent ratio. Record the weight (to the nearest mg) of a collection vessel with cap and extracting solvent. Place the collection vessel under the generator column so that water from the collecting tube enters below the level of the extracting solvent (figure 2 in paragraph (c)(1)(i)(A)(2) of this section). When the collection vessel is filled, remove it from under the generator column, replace cap, and weigh the filled vessel. Determine the weight of water collected. Before analyzing for the solute, gently shake the collection vessel contents for Start Printed Page 78771approximately 30 min, controlling the rate of shaking so as not to form an emulsion; rotating the flask end over end five times per minute is sufficient.
(4) Analysis of the solute. (i) After shaking, allow the collection vessel to stand for approximately 30 min; then remove a known volume of the extracting solvent from the vessel using a microliter syringe and inject it into the GC. Record the ratio of peak area to volume injected and, from the regression equation of the calibration line, determine the concentration of solute in the extracting solvent. In this equation, Ces is the concentration of solute in extracting solvent (M), dH2O and des are the densities of water and extracting solvent, respectively, and ges and gH2O are the grams of extracting solvent and water, respectively, contained in the collection vessel. The concentration of solute in water C(M) is determined from the following equation:
Equation 5:
(ii) Make replicate injections from each collecting vessel to determine the average solute concentration in water for each vessel. To make sure the generator column has reached equilibrium, run at least two additional (for a total of three) collection vessels and analyze the extracted solute as described above. Calculate the water solubility of the solute from the average solute concentration in the three vessels.
(iv) Modification of procedures for potential problems. If the test compound decomposes in one or more of the aqueous solvents required during the period of the test at a rate such that an accurate value for water solubility cannot be obtained, then it will be necessary to carry out detailed transformation studies; e.g., hydrolysis in paragraph (e)(16) of this section. If decomposition is due to aqueous photolysis, then it will be necessary to carry out water solubility studies in the dark, under red or yellow lights, or by any other suitable method to eliminate this transformation process.
(d) Data and reporting— (1) Test report. (i) For each set of conditions, (e.g., temperature, pure water, buffer solution, artificial seawater) required for the study, provide the water solubility value for each of three determinations, the mean value, and the standard deviation.
(ii) For compounds that decompose at a rate such that a precise value for the water solubility cannot be obtained, provide a statement to that effect.
(iii) For compounds with water solubility below 1 ppb, report the value as “less than 1 ppb.”
(2) Specific analytical, calibration, and recovery procedures. (i) For the HPLC method describe and/or report:
(A) The method used to determine the sample-loop volume and the average and standard deviation of that volume.
(B) The average and standard deviation of the RF.
(C) Any changes made or problems encountered in the test procedure.
(ii) For the GC, or any other analytical, method report:
(A) The column and GC operating conditions of temperature and flow rate, or the operating conditions of any other analytical method used.
(B) The average and standard deviation of the average area per microliter obtained for each of the standard solutions.
(C) The form of the regression equation obtained in the calibration procedure.
(D) The extracting solvent used, and its extraction efficiency.
(E) The average and standard deviation of solute concentration in each collection vessel.
(F) Any changes made or problems encountered in the test procedure.
(G) If applicable, a complete description of the analytical method which was used instead of the GC method.
(e) References. For additional information on this test guideline, the following references should be consulted. These references are available from the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., SW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, excluding legal holidays.
(1) DeVoe, H. et al., Generator columns and high pressure liquid chromatography for determining aqueous solubilities and octanol-water partition coefficients of hydrophobic substances. Journal of Research, National Bureau of Standards, 86:361-366 (1981).
(2) Hansch, C. et al., The linear free-energy relationship between partition coefficients, and the aqueous solubility of organic liquids. Journal of Organic Chemistry 33:347-350 (1968).
(3) Leifer, A. et al., Environmental transport and transformation of polychlorinated biphenyls. Chapter 1. U.S. Environmental Protection Agency Report: EPA-560/5-83-005 (1983).
(4) Mackay, D. et al., Relationships between aqueous solubility and octanol-water partition coefficient. Chemosphere 9:701-711 (1980).
(5) May, W.E. et al., Determination of the aqueous solubility of polynuclear aromatic hydrocarbons by a coupled column liquid chromatographic technique. Analytical Chemistry 50:175-179 (1978).
(6) May, W.E. et al. Determination of the solubility behavior of some polycyclic aromatic hydrocarbons in the water. Analytical Chemistry, 50:997-1000 (1978a).
(7) Miller, N.M. et al., Aqueous solubilities, octanol/water partition coefficients, and entropy of melting of chlorinated benzenes and biphenyls. Journal of Chemical and Engineering Data 29:184-190 (1984).
(8) OECD/Organization for Economic Cooperation and Development. Test Guideline No. 105. Water solubility column elution-flask method (1981).
(9) Sutton, C. and Calder, J.A., Solubility of alkylbenzenes in distilled water and seawater at 25 °C. Journal of Chemical and Engineering Data 20:320-322 (1975).
(10) Tewari, Y.B. et al., Aqueous solubility and octanol/water partition coefficient of organic compounds at 25 °C. Journal of Chemical and Engineering Data 27:451-454 (1982).
(11) Wasik, S.P. et al., Octanol/Water Partition Coefficient and Aqueous Solubilities of Organic Compounds. NBS Report NBSIR 81-2406. Washington, DC: National Bureau of Standards, U.S. Department of Commerce (1981).
(12) Yalkowski, S.H. et al., “Aquasol database of aqueous solubilities of organic compounds”; Fifth Edition. University of Arizona, College of Pharmacy, Tucson, AZ 85721 (1990) (available at http://www.pharm.arizona.edu/aquasol/index.html).
(13) ASTM D 1193-91, Standard Specification for Reagent Water. American Society for Testing and Materials (ASTM). 1916 Race St., Philadelphia, PA 19103.
Subpart H—[Amended]
3. Sections 799.9110, 799.9120, 799.9130, 799.9305, 799.9310, 799.9325, 799.9355, 799.9365, 799.9410, 799.9430, 799.9537, 799.9630, and 799.9748 are added to subpart H to read as follows:
TSCA acute oral toxicity.(a) Scope. This section is intended to meet the testing requirements under section 4 of the Toxic Substances Control Act (TSCA). In the assessment and evaluation of the toxic characteristics of a substance, determination of acute oral toxicity is usually an initial step. It provides Start Printed Page 78772information on health hazards likely to arise from short-term exposure by the oral route. Data from an acute study may serve as a basis for classification and labeling. It is traditionally a step in establishing a dosage regimen in subchronic and other studies and may provide initial information on the mode of toxic action of a substance. An evaluation of acute toxicity data should include the relationship, if any, between the exposure of animals to the test substance and the incidence and severity of all abnormalities, including behavioral and clinical abnormalities, the reversibility of observed abnormalities, gross lesions, body weight changes, effects on mortality, and any other toxic effects.
(b) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides, and Toxic Substances (OPPTS) harmonized test guideline 870.1100 (August 1998, final guideline). This source is available at the address in paragraph (f) of this section.
(c) Definitions. The following definitions apply to this section.
Acute oral toxicity is the adverse effects occurring within a short period of time after oral administration of either a single dose of a substance or multiple doses given within a 24-hour period.
Dosage is a general term comprising the dose, its frequency, and the duration of dosing.
Dose is the amount of test substance administered. Dose is expressed as weight of test substance (milligrams, grams) per unit weight of test animal (e.g., milligrams per kilogram).
Dose-effect is the relationship between the dose and the magnitude of a defined biological effect either in an individual or in a population sample.
Dose-response is the relationship between the dose and the proportion of a population sample showing a defined effect.
LD50 (median lethal dose) is a statistically derived estimate of single dose of a substance that can be expected to cause death in 50% of animals when administered by the oral route. The LD50 value is expressed in terms of weight of test substance per unit weight of test animal (milligrams per kilogram).
(d) Alternative approaches to the determination of acute toxicity. (1) EPA will accept the following procedures to reduce the number of animals used to evaluate acute effects of chemical exposure while preserving its ability to make reasoned judgments about safety:
(i) Estimation of acute oral toxicity. When further study is warranted, EPA generally supports limiting such tests to those using the lowest number of animals feasible. EPA will accept three alternative Organization for Economic Cooperation and Development (OECD) test methods in place of the “traditional” acute oral toxicity test. The three OECD alternatives are the following:
(A) The up and down procedure as described in OECD Guideline 425 referenced in paragraph (f)(4) of this section.
(B) The acute toxic class method as described in OECD Guideline 423 and referenced in paragraph (f)(6) of this section.
(C) The fixed dose method as described in OECD Guideline 420 and referenced in paragraph (f)(5) of this section.
(ii) Limit test. When data on structurally related chemicals are inadequate, a limit test may be considered. If rodents are used, a limit dose of at least 2,000 mg per kilogram of body weight may be administered to a single group of five males and five females using the procedures described in paragraph (e) of this section. If no lethality is demonstrated, no further testing for acute oral toxicity is needed. (Under current policy and regulations for pesticide products, precautionary statements may still be required unless there are data to indicate the LD50 is greater than 5,000 mg/kg.) If compound-related mortality is produced in the limit test, further study may need to be considered.
(2) [Reserved]
(e) Conventional acute toxicity test—(1) Principle of the test method. The test substance is administered orally by gavage in graduated doses to several groups of experimental animals, one dose being used per group. The doses chosen may be based on the results of a range finding test. Subsequently, observations of effects and deaths are made. Animals that die during the test are necropsied, and at the conclusion of the test the surviving animals are sacrificed and necropsied. This section is directed primarily to studies in rodent species but may be adapted for studies in nonrodents. Animals showing severe and enduring signs of distress and pain may need to be humanely sacrificed. Dosing test substances in a way known to cause marked pain and distress due to corrosive or irritating properties need not be carried out.
(2) Substance to be tested. Test, control, and reference substances are described in 40 CFR Part 792—Good Laboratory Practice Standards.
(3) Test procedures—(i) Preparations. Healthy young adult animals are acclimatized to the laboratory conditions for at least 5 days prior to the test before the test animals are randomized and assigned to the treatment groups.
(ii) Animal selection—(A) Species and strain. Although several mammalian test species may be used, the rat is the preferred species. Commonly used laboratory strains must be employed. If another species is used, the tester must provide justification and reasoning for its selection.
(B) Age. Young adult rats between 8- and 12-weeks-old at the beginning of dosing should be used. Rabbits should be at least 12 weeks of age at study initiation. The weight variation of animals used in a test must be within 20% of the mean weight for each sex.
(C) Number and sex of animals. (1) At least five experimentally naive rodents are used at each dose level. They should all be of the same sex. After completion of the study in one sex, at least one group of five animals of the other sex is dosed to establish that animals of this sex are not markedly more sensitive to the test substance. The use of fewer animals may be justified in individual circumstances. Where adequate information is available to demonstrate that animals of the sex tested are markedly more sensitive, testing in animals of the other sex may be dispensed with. An acceptable option would be to test at least one group of five animals per sex at one or more dose levels to definitively determine the more sensitive sex prior to conducting the main study.
(2) The females must be nulliparous and nonpregnant.
(3) In acute toxicity tests with animals of a higher order than rodents, the use of smaller numbers should be considered.
(D) Assignment of animals. Each animal must be assigned a unique identification number. A system to assign animals to test groups and control groups randomly is required.
(E) Housing. Animals may be group-caged by sex, but the number of animals per cage must not interfere with clear observation of each animal. The biological properties of the test substance or toxic effects (e.g., morbidity, excitability) may indicate a need for individual caging.
(1) The temperature of the experimental animal rooms should be at 22 ± 3 °C for rodents.
(2) The relative humidity of the experimental animal rooms should be 30 to 70%.
(3) Where lighting is artificial, the sequence should be 12-hours light/12-hours dark. Start Printed Page 78773
(4) For feeding, conventional laboratory diets may be used with an unlimited supply of drinking water.
(iii) Dose levels and dose selection. (A) Three dose levels must be used, spaced appropriately to produce test groups with a range of toxic effects and mortality rates. The data collected must be sufficient to produce a dose-response curve and permit an acceptable estimation of the LD50. Range finding studies using single animals may help to estimate the positioning of dose groups so that no more than three dose levels will be necessary.
(B) Limit test. This test has been defined and described in paragraph (d)(1)(ii) of this section.
(C) Vehicle. Where necessary, the test substance is dissolved or suspended in a suitable vehicle. If a vehicle or diluent is needed, it should not elicit toxic effects itself nor substantially alter the chemical or toxicological properties of the test substance. It is recommended that wherever possible the use of an aqueous solution be considered first, followed by consideration of a solution in oil (e.g., corn oil), and then by consideration of possible solution in other vehicles. Toxic characteristics of nonaqueous vehicles should be known, and, if not known, should be determined before the test.
(D) Volume. The maximum volume of liquid that can be administered at one time depends on the size of the test animal. In rodents, the volume should not exceed 1 mL/100 g body weight, except when an aqueous solution is used in which case 2 mL/100 g may be administered. Either constant volume or constant concentration administration is acceptable when dosing, provided the following guidance is employed. When possible, the liquid test material should be dosed neat. Otherwise, it may be diluted, using the highest concentration possible, although volumes less than 0.5 mL per animal would not be required. Lower dose volumes are acceptable if they can be accurately administered. Solid materials should be suspended or dissolved in the minimum amount of vehicle and dosed at the highest concentration possible.
(iv) Exposure and exposure duration. (A) Animals must be fasted prior to test substance administration. For the rat, feed should be withheld overnight; for other rodents with higher metabolic rates a shorter period of fasting is appropriate.
(B) The test substance must be administered in a single dose by gavage, using a stomach tube or suitable intubation cannula.
(C) If a single dose is not possible, the dose may be given in smaller fractions over a period not exceeding 24 hours. Where a dose is administered in fractions, it may be necessary to provide the animals with food and water, depending on the length of the dosing period.
(D) After the substance has been administered, feed may be withheld for an additional 3-4 hours.
(v) Observation period. Although 14 days is recommended as a minimum observation period, the duration of observation should not be fixed rigidly. It should be determined by the toxic reactions, rate of onset, and length of recovery period, and may thus be extended when considered necessary. The time at which signs of toxicity appear, their duration, and the time to death are important, especially if there is a tendency for deaths to be delayed.
(vi) Observation of animals. (A) A careful clinical examination must be made at least once each day.
(B) Additional observations must be made daily, especially in the early days of the study. Appropriate actions should be taken to minimize loss of animals to the study (e.g., necropsy or refrigeration of those animals found dead and isolation of weak or moribund animals).
(C) Observations must be detailed and carefully recorded, preferably using explicitly defined scales. Observations should include, but not be limited to, evaluation of skin and fur, eyes and mucous membranes, respiratory and circulatory effects, autonomic effects such as salivation, central nervous system effects, including tremors and convulsions, changes in the level of activity, gait and posture, reactivity to handling or sensory stimuli, altered strength, and stereotypies or bizarre behavior (e.g., self-mutilation, walking backwards).
(D) Individual weights of animals must be determined shortly before the test substance is administered, weekly thereafter, and at death. Changes in weights should be calculated and recorded when survival exceeds 1 day.
(E) The time of death should be recorded as precisely as possible.
(vii) Gross pathology. (A) At the end of the test, surviving animals must be weighed and sacrificed.
(B) A gross necropsy must be performed on all animals under test. All gross pathology changes should be recorded.
(C) If necropsy cannot be performed immediately after a dead animal is discovered, the animal should be refrigerated (not frozen) at temperatures low enough to minimize autolysis. Necropsies should be performed as soon as practicable, normally within a day or two.
(viii) Additional evaluation. Microscopic examination of organs showing evidence of gross pathology in animals surviving 24 hours or more should also be considered because it may yield useful information.
(ix) Data and reporting—(A) Treatment of results. Data must be summarized in tabular form, showing for each test group the number of animals at the start of the test, body weights, time of death of individual animals at different dose levels, number of animals displaying other signs of toxicity, description of toxic effects, and necropsy findings. Any methods used for calculation of the LD50 or any other parameters should be specified and referenced. Methods for parameter estimation are described in the references listed in paragraphs (f)(1), (f)(2), and (f)(3) of this section.
(B) Evaluation of results. An evaluation should include the relationship, if any, between exposure of the animals to the test substance and the incidence and severity of all abnormalities, including behavioral and clinical abnormalities, gross lesions, body weight changes, effects on mortality, and any other toxic effects. The LD50 value should always be considered in conjunction with the observed toxic effects and any necropsy findings. The LD50 value is a relatively coarse measurement, useful only as a reference value for classification and labeling purposes, and for an expression of the lethal potential of the test substance by the ingestion route. Reference should always be made to the experimental animal species in which the LD50 value was obtained.
(C) Test report. In addition to the reporting requirements specified under EPA Good Laboratory Practice Standards at 40 CFR part 792, subpart J, the following specific information must be reported. The test report shall include:
(1) Species, strain, sex, and source of test animals.
(2) Method of randomization in assigning animals to test and control groups.
(3) Rationale for selection of species, if other than that recommended.
(4) Tabulation of individual and test group data by sex and dose level (e.g., number of animals exposed, number of animals showing signs of toxicity and number of animals that died or were sacrificed during the test).
(i) Description of toxic effects, including their time of onset, duration, reversibility, and relationship to dose.
(ii) Body weights.
(iii) Time of dosing and time of death after dosing. Start Printed Page 78774
(iv) Dose-response curves for mortality and other toxic effects (when permitted by the method of determination).
(v) Gross pathology findings.
(vi) Histopathology findings and any additional clinical chemistry evaluations, if performed.
(5) Description of any pretest conditioning, including diet, quarantine and treatment for disease.
(6) Description of caging conditions including: Number (or change in number) of animals per cage, bedding material, ambient temperature and humidity, photoperiod, and identification of diet of test animals.
(7) Manufacturer, source, purity, and lot number of test substance.
(8) Relevant properties of substance tested including physical state and pH (if applicable).
(9) Identification and composition of any vehicles (e.g., diluents, suspending agents, and emulsifiers) or other materials used in administering the test substance.
(10) A list of references cited in the body of the report. References to any published literature used in developing the test protocol, performing the testing, making and interpreting observations, and compiling and evaluating the results.
(f) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., NW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Chanter, D.O. and Heywood, R. The LD50 Test: Some Considerations of Precision. Toxicology Letters 10:303-307 (1982).
(2) Finney, D.J. Chapter 3—Estimation of the median effective dose and Chapter 4—Maximum likelihood estimation, Probit Analysis, 3rd ed. Cambridge, London (1971).
(3) Finney, D.J. The Median Lethal Dose and Its Estimation. Archives of Toxicology 56:215-218 (1985).
(4) Organization for Economic Cooperation and Development. OECD Guidelines for the Testing of Chemicals. OECD Guideline 425: Acute Oral Toxicity: Up-and-Down Procedure, Approved: June 1998.
(5) Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals. Guideline 420: Acute Oral Toxicity—Fixed Dose Method, Adopted: July 17, 1992.
(6) Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals. Guideline 423: Acute Oral Toxicity—Acute Toxic Class Method, Adopted: March 22, 1996.
(7) Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals. Guideline 401: Acute Oral Toxicity, Adopted: February 24, 1987.
TSCA acute dermal toxicity.(a) Scope. This section is intended to meet the testing requirements under section 4 of the Toxic Substances Control Act (TSCA). In the assessment and evaluation of the toxic characteristics of a substance, determination of acute dermal toxicity is useful where exposure by the dermal route is likely. It provides information on health hazards likely to arise from short-term exposure by the dermal route. Data from an acute study may serve as a basis for classification and labeling. It is an initial step in establishing a dosage regimen in subchronic and other studies and may provide information on dermal absorption and the mode of toxic action of a substance by this route. An evaluation of acute toxicity data should include the relationship, if any, between the exposure of animals to the test substance and the incidence and severity of all abnormalities, including behavioral and clinical abnormalities, the reversibility of observed abnormalities, gross lesions, body weight changes, effects on mortality, and any other toxic effects.
(b) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides, and Toxic Substances (OPPTS) harmonized test guideline 870.1200 (August 1998, final guideline). This source is available at the address in paragraph (f) of this section.
(c) Definitions. The following definitions apply to this section.
Acute dermal toxicity is the adverse effects occurring within a short time of dermal application of a single dose of a substance or multiple doses given within a 24-hour period.
Dosage is a general term comprising the dose, its frequency and the duration of dosing.
Dose is the amount of test substance applied. Dose is expressed as weight of test substance (grams, milligrams) per unit weight of test animal (e.g., milligrams per kilogram).
Dose-effect is the relationship between the dose and the magnitude of a defined biological effect either in an individual or in a population sample.
Dose-response is the relationship between the dose and the proportion of a population sample showing a defined effect.
LD50 (median lethal dose), dermal, is a statistically derived estimate of a single dose of a substance that can be expected to cause death in 50% of treated animals when applied to the skin. The LD50 value is expressed in terms of weight of test substance per unit weight of test animal (milligrams per kilogram).
(d) Approaches to the determination of acute toxicity. (1) EPA recommends the following means to reduce the number of animals used to evaluate acute effects of chemical exposure while preserving its ability to make reasonable judgments about safety:
(i) Using data from substantially similar mixtures. In order to minimize the need for animal testing, the Agency encourages the review of existing acute toxicity information on mixtures that are substantially similar to the mixture under investigation. In certain cases it may be possible to glean enough information to make preliminary hazard evaluations that may reduce the need for further animal testing.
(ii) Limit test. When data on structurally related chemicals are inadequate, a limit test may be considered. If rodents are used, a limit dose of at least 2,000 mg/kg bodyweight may be administered to a single group of five males and five females using the procedures described in paragraph (e) of this section. If no lethality is demonstrated, no further testing for acute dermal toxicity is needed. If compound-related mortality is produced, further study may need to be considered.
(2) [Reserved]
(e) Conventional acute toxicity test—(1) Principle of the test method. The test substance is applied dermally in graduated doses to several groups of experimental animals, one dose being used per group. The doses chosen may be based on the results of a range finding test. Subsequently, observations of effects and deaths are made. Animals that die during the test are necropsied, and at the conclusion of the test the surviving animals are sacrificed and necropsied. This section is directed primarily to studies in either rats, rabbits, or guinea pigs but may be adapted for studies in other species. Animals showing severe and enduring signs of distress and pain may need to be humanely sacrificed. Dosing test substances in a way known to cause marked pain and distress due to corrosive or irritating properties need not be carried out. Start Printed Page 78775
(2) Substance to be tested. Test, control, and reference substances are discussed in 40 CFR Part 792—Good Laboratory Practice Standards.
(3) Test procedures—(i) Preparations. Healthy young adult animals are acclimatized to the laboratory conditions for at least 5 days prior to the test before the test animals are randomized and assigned to the treatment groups.
(ii) Animal selection—(A) Species and strain. The rat, rabbit, or guinea pig may be used. The albino rabbit is preferred because of its size, ease of handling, skin permeability, and extensive data base. Commonly used laboratory strains must be employed. If a species other than rats, rabbits, or guinea pigs is used, the tester must provide justification and reasoning for its selection.
(B) Age. Young adult animals, rats between 8- and 12-weeks-old, rabbits at least 12-weeks-old, and guinea pigs between 5- and 6-weeks-old at the beginning of dosing should be used. The weight variation of animals used in a test must be within 20% of the mean weight for each sex.
(C) Number and sex of animals. (1) At least five experimentally naive animals with healthy intact skin are used at each dose level. They should all be of the same sex. After completion of the study in one sex, at least one group of five animals of the other sex is dosed to establish that animals of this sex are not markedly more sensitive to the test substance. The use of fewer animals may be justified in individual circumstances. Where adequate information is available to demonstrate that animals of the sex tested are markedly more sensitive, testing in animals of the other sex may be dispensed with. An acceptable option would be to test at least one group of five animals per sex at one or more dose levels to definitively determine the more sensitive sex prior to conducting the main study.
(2) The females must be nulliparous and nonpregnant.
(3) In acute toxicity tests with animals of a higher order than those mentioned above, the use of smaller numbers should be considered.
(D) Assignment of animals. Each animal must be assigned a unique identification number. A system to randomly assign animals to test groups and control groups is required.
(E) Housing. Animals should be housed in individual cages.
(1) The temperature of the experimental animal rooms should be at 22 ± 3 °C for rodents, 20 ± 3 °C for rabbits.
(2) The relative humidity of the experimental animal rooms should be 30 to 70%.
(3) Where lighting is artificial, the sequence should be 12-hours light/12-hours dark.
(4) For feeding, conventional laboratory diets may be used with an unlimited supply of drinking water.
(iii) Dose levels and dose selection. (A) Three dose levels must be used and spaced appropriately to produce test groups with a range of toxic effects and mortality rates. The data must be sufficient to produce a dose-response curve and permit an acceptable estimation of the median lethal dose. Range finding studies using single animals may help to estimate the positioning of the dose groups so that no more than three dose levels will be necessary.
(B) Limit test. This test is described in paragraph (d)(2)(ii) of this section.
(C) Vehicle. Solids should be pulverized when possible. The test substance should be moistened sufficiently with water or, where necessary, a suitable vehicle to ensure good contact with skin. If a vehicle or diluent is needed, it should not elicit toxic effects itself nor substantially alter the chemical or toxicological properties of the test substance. In addition, the influence of the vehicle on penetration of skin by the test substance should be taken into account. It is recommended that wherever possible the use of an aqueous solution be considered first, followed by consideration of a solution in oil (e.g., corn oil), and then by consideration of possible solution in other vehicles. For nonaqueous vehicles the toxic characteristics of the vehicle should be known, and if not known should be determined before the test. Acceptable alternative vehicles include gum arabic, ethanol and water, carboxymethyl cellulose, glycerol, propylene glycol, PEG vegetable oil, and mineral oil as long as the vehicle is not irritating and the inability to use water or saline is justified in the report.
(iv) Exposure and exposure duration. The test substance must be administered over a period of 24 hours.
(v) Preparation of animal skin. Fur must be clipped from the dorsal area of the trunk of the test animals. Shaving may be employed, but it should be carried out at least 24 hours before dosing. Care must be taken to avoid abrading the skin, which would alter its permeability.
(vi) Application of test substance. (A) The test substance must be applied uniformly over a shaved or clipped area which is approximately 10% of the body surface area. The area starting at the scapulae (shoulders) to the wing of the ileum (hip bone) and half way down the flank on each side of the animal should be shaved or clipped. Liquid test materials should be undiluted if possible. With highly toxic substances, the surface area covered may be less, but as much of the area as possible should be covered with as thin and uniform a film as practical. The test material is not removed until 24 hours after application. In the case where less than 10% of the surface area is covered an approximation of the exposed areas should be determined.
(B) The test substance must be held in contact with the skin with a porous gauze dressing (<8 ply) and nonirritating tape throughout a 24-hour exposure period. The test site must be further covered in a suitable manner to retain the gauze dressing and test substance and ensure that the animals cannot ingest the test substance. Restrainers may be used to prevent the ingestion of the test substance, but complete immobilization is not a recommended method. Although a semiocclusive dressing is preferred, an occlusive dressing will also be acceptable.
(C) At the end of the exposure period, residual test substance should be removed where practicable using water or an appropriate solvent.
(vii) Observation period. Although 14 days is recommended as a minimum observation period, the duration of observation should not be fixed rigidly. It should be determined by the toxic reactions, rate of onset, and length of recovery period, and may thus be extended when considered necessary. The time at which signs of toxicity appear, their duration, and the time to death are important, especially if there is a tendency for deaths to be delayed.
(viii) Observation of animals. (A) A careful clinical examination must be made at least once each day.
(B) Additional observations must be made daily, especially in the early days of the study. Appropriate actions should be taken to minimize loss of animals to the study (e.g., necropsy or refrigeration of those animals found dead and isolation of weak or moribund animals).
(C) Observations must be detailed and carefully recorded, preferably using explicitly defined scales. Observations should include, but not be limited to, evaluation of skin and fur, eyes and mucous membranes, respiratory and circulatory effects, autonomic effects such as salivation, central nervous system effects, including tremors and convulsions, changes in the level of activity, gait and posture, reactivity to handling or sensory stimuli, altered Start Printed Page 78776strength, and stereotypies or bizarre behavior (e.g., self-mutilation, walking backwards).
(D) Individual weights of animals must be determined shortly before the test substance is administered, weekly thereafter, and at death. Changes in weights should be calculated and recorded when survival exceeds one day.
(E) The time of death should be recorded as precisely as possible.
(ix) Gross pathology. (A) At the end of the test, surviving animals must be weighed and sacrificed.
(B) A gross necropsy must be performed on all animals under test. All gross pathology changes should be recorded.
(C) If necropsy cannot be performed immediately after a dead animal is discovered, the animal should be refrigerated (not frozen) at temperatures low enough to minimize autolysis. Necropsies should be performed as soon as practicable, normally within a day or two.
(x) Additional evaluations. Microscopic examination of organs showing evidence of gross pathology in animals surviving 24 hours or more should also be considered because it may yield useful information.
(xi) Data and reporting—(A) Treatment of results. Data must be summarized in tabular form, showing for each test group the number of animals at the start of the test, body weights, time of death of individual animals at different dose levels, number of animals displaying other signs of toxicity, description of toxic effects and necropsy findings. Any methods used for calculation of the LD50 or any other parameters should be specified and referenced. Methods for parameter estimation are described in the references listed in paragraphs (f)(1), (f)(2), and (f)(3) of this section.
(B) Evaluation of results. An evaluation should include the relationship, if any, between exposure of the animals to the test substance and the incidence and severity of all abnormalities, including behavioral and clinical abnormalities, gross lesions, body weight changes, effects on mortality, and any other toxic effects. The LD50 value should always be considered in conjunction with the observed toxic effects and any necropsy findings. The LD50 value is a relatively coarse measurement, useful only as a reference value for classification and labeling purposes, and for an expression of the lethal potential of the test substance by the dermal route. Reference should always be made to the experimental animal species in which the LD50 value was obtained.
(C) Test report. In addition to the reporting requirements specified under EPA Good Laboratory Practice Standards at 40 CFR part 792, subpart J, the following specific information must be reported. The test report must include:
(1) Species, strain, sex, and source of test animals.
(2) Method of randomization in assigning animals to test and control groups.
(3) Rationale for selection of species, if other than that recommended.
(4) Tabulation of individual and test group data by sex and dose level (e.g., number of animals exposed, number of animals showing signs of toxicity and number of animals that died or were sacrificed during the test).
(i) Description of toxic effects, including their time of onset, duration, reversibility, and relationship to dose.
(ii) Body weights.
(iii) Time of dosing and time of death after dosing.
(iv) Dose-response curves for mortality and other toxic effects (when permitted by the method of determination).
(v) Gross pathology findings.
(vi) Histopathology findings and any additional clinical chemistry evaluations, if performed.
(5) Description of any pre-test conditioning, including diet, quarantine and treatment for disease.
(6) Description of caging conditions including: Number (or change in number) of animals per cage, bedding material, ambient temperature and humidity, photoperiod, and identification of diet of test animals.
(7) Manufacturer, source, purity, and lot number of test substance.
(8) Relevant properties of substance tested including physical state and pH (if applicable).
(9) Identification and composition of any vehicles (e.g., diluents, suspending agents, and emulsifiers) or other materials used in administering the test substance.
(10) A list of references cited in the body of the report. References to any published literature used in developing the test protocol, performing the testing, making and interpreting observations, and compiling and evaluating the results.
(f) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., NW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Chanter, D.O. and Heywood, R., The LD50 Test: Some Considerations of Precision, Toxicology Letters 10:303-307 (1982).
(2) Finney, D.J. Chapter 3—Estimation of the median effective dose and Chapter 4-Maximum likelihood estimation, Probit Analysis, 3rd ed. Cambridge, London (1971).
(3) Finney, D.J. The Median Lethal Dose and Its Estimation. Archives of Toxicology 56:215-218 (1985).
(4) Organization for Economic Cooperation and Development. OECD Guideline for the Testing of Chemicals. OECD Guideline 425: Acute Oral Toxicity: Up-and-Down Procedure. Adopted: September 21, 1998.
(5) Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals. Guideline 420: Acute Oral Toxicity—Fixed Dose Method. Adopted: July 17, 1992.
(6) Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals. Guideline 423: Acute Oral Toxicity—Acute Toxic Class Method. Adopted: March 22, 1996
(7) Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals. Guideline 402: Acute Dermal Toxicity. Adopted: February 24, 1987.
TSCA acute inhalation toxicity.(a) Scope. This section is intended to meet testing requirements under section 4 of the Toxic Substances Control Act (TSCA). Determination of acute toxicity is usually an initial step in the assessment and evaluation of the toxic characteristics of a substance that may be inhaled such as a gas, volatile substance, or aerosol/particle. It provides information on health hazards likely to arise from short-term exposure by the inhalation route. Data from an acute study may serve as a basis for classification and labeling. It is traditionally a step in establishing a dosage regimen in subchronic and other studies and may provide initial information on the mode of toxic action of a substance. An evaluation of acute toxicity data should include the relationship, if any, between the animals' exposure to the test substance and the incidence and severity of all abnormalities, including behavioral and clinical abnormalities, the reversibility of observed abnormalities, gross lesions, body weight changes, effects on mortality, and any other toxic effects. Start Printed Page 78777
(b) Source. The source material used in developing this TSCA test guideline is the harmonized Office of Prevention, Pesticides, and Toxic Substances (OPPTS) test guideline 870.1300 (August 1998, final guideline). These sources are available at the address in paragraph (g) of this section.
(c) Definitions. The definitions in section 3 of TSCA and the definitions in 40 CFR Part 792—Good Laboratory Practice Standards apply to this section. The following definitions also apply to this section.
Acute inhalation toxicity is the adverse effect caused by a substance following a single uninterrupted exposure by inhalation over a short period of time (24 hours or less) to a substance capable of being inhaled.
Aerodynamic equivalent diameter is defined as the diameter of a unit-density sphere having the same terminal settling velocity as the particle in question, whatever its size, shape, and density. It is used to predict where in the respiratory tract such particles may be deposited.
Concentration is expressed as weight of the test substance per unit volume of air, e.g., milligrams per liter.
Geometric standard deviation (GSD) is a dimensionless number equal to the ratio between the mass median aerodynamic diameter (MMAD) and either 84% or 16% of the diameter size distribution (e.g., MMAD = 2 m; 84% = 4 m; GSD = 4/2 = 2.0.) The MMAD, together with the GSD, describe the particle size distribution of an aerosol. Use of the GSD may not be valid for non-lognormally distributed aerosols. (If the size distribution deviates from the lognormal, it shall be noted).
Inhalable diameter refers to that aerodynamic diameter of a particle which is considered to be inhalable for the organism under study. It is used to refer to particles which are capable of being inhaled and deposited anywhere within the respiratory tract .
LC50 (median lethal concentration) is a statistically derived estimate of a concentration of a substance that can be expected to cause death during exposure or within a fixed time after exposure in 50% of animals exposed for a specified time. The LC50 value is a relatively coarse measurement useful only for classification and labeling purposes and an expression of the lethal potential of the test substance following inhalation. The LC50 value is expressed as weight of test substance per unit volume of air (milligrams per liter) or parts per million. For clarity, the exposure duration and test animal species should also be specified, e.g., 4 hours LC50 in F344.
Mass median aerodynamic diameter (MMAD) is the median aero-dynamic diameter and, along with the geometric standard deviation, is used to describe the particle size distribution of any aerosol statistically, based on the weight and size of the particles. Fifty percent of the particles by weight will be smaller than the median diameter and 50% of the particles will be larger.
(d) Approaches to the determination of acute toxicity. (1) EPA recommends the following means to reduce the number of animals used to evaluate acute effects of chemical exposure while preserving its ability to make reasonable judgments about safety:
(i) Using data from substantially similar mixtures. In order to minimize the need for animal testing, the Agency encourages the review of existing acute toxicity information on mixtures that are substantially similar to mixtures under investigation. In certain cases, it may be possible to get enough information to make preliminary hazard evaluations that may reduce the need for further animal testing.
(ii) Limit test. When data on structurally related chemicals are inadequate, a limit test may be considered. In the limit test, a single group of five males and five females is exposed to 2 mg/L for 4 hours, or where this is not possible due to physical or chemical properties of the test substance, the maximum attainable concentration where a particle size distribution having an MMAD between 1 and 4 mm cannot be maintained, using the procedures described under paragraph (e) of this section. For fibers, the bivariate distribution of length and diameter must ensure inhalability. For gases and vapors, the concentrations need not be greater than 50,000 ppm or 50% of the lower explosive limit, whichever is lower. If a test at an aerosol or particulate exposure of 2 mg/L (actual concentration of respirable substance) for 4 hours or, where this is not feasible, the maximum attainable concentration, using the procedures described for this study, produces no observable toxic effects, then a full study using three concentrations will not be necessary. Similarly, if a test at a gas or vapor exposure of 50,000 ppm or 50% of the lower explosive limit, whichever is lower, produces no observable toxic effects, then a full study using three concentrations will not be necessary.
(2) [Reserved]
(e) Conventional acute toxicity test—(1) Principle of the test method. Several groups of experimental animals are exposed to the test substance in graduated concentrations for a defined period, one concentration being used per group. When a vehicle other than water is used to help generate an appropriate concentration of the substance in the atmosphere, a vehicle control group should be used when historical data are not available or adequate to determine the acute inhalation toxicity of the vehicle. Subsequently, observations of effects and death are made. Animals that die during the test are necropsied and at the conclusion of the test surviving animals are sacrificed and necropsied. This guideline is directed primarily to studies in rodent species but may be adapted for studies in non-rodents. Animals showing severe and enduring signs of distress and pain may need to be sacrificed. Dosing test substances in a way known to cause marked pain and distress due to corrosive or irritating properties need not be carried out.
(2) Substance to be tested. Test, control, and reference substances are discussed under EPA Good Laboratory Practice Standards at 40 CFR part 792, subpart f.
(3) Test procedures—(i) Preparation. Healthy young adult animals are acclimatized to the laboratory conditions for at least 5 days prior to the test. Before the test, animals are randomized and assigned to the required number of groups.
(ii) Animal selection—(A) Species and strain. (1) Although several mammalian test species may be used, the preferred species is the rat. Commonly used laboratory strains should be employed. If another mammalian species is used, the investigator should provide justification and reasoning for the selection.
(2) Health Status. Body weight and feed consumption are not sufficient indicators of the health status of animals prior to initiating an inhalation toxicity study. Prior to initiating the study, animals must be monitored for known viral and bacterial respiratory pathogens determined by conventional microbiological assays (e.g., serology). The animals must be free from pathogens at the start of exposure.
(B) Age. Young adult rats between 8-12 weeks old at the beginning of dosing, should be used. The weight variation in animals or between groups used in a test should not exceed ± 20% of the mean weight of each sex.
(C) Number of animals and sex. (1) At least five experimentally naive animals are used at each concentration and they must be of one sex. After completion of the study in one sex, at least one group of five animals of the other sex is exposed to establish that animals of this sex are not markedly more sensitive to Start Printed Page 78778the test substance. The use of fewer animals may be justified in individual circumstances. Where adequate information is available to demonstrate that animals of the sex tested are markedly more sensitive, testing in animals of the other sex is not required. An acceptable option would be to test at least one group of five animals per sex at one or more dose levels to definitively determine the more sensitive sex prior to conducting the main study.
(2) Females must be nulliparous and nonpregnant.
(3) In acute toxicity tests with animals of a higher order than rodents, the use of fewer animals per concentration group should be considered.
(D) Assignment of animals. (1) Each animal must be assigned a unique identification number. A system to assign animals to test groups and control groups randomly is required.
(2) Control groups. A concurrent untreated control group is not necessary. Where a vehicle other than water is used to generate an appropriate concentration of the test substance in the atmosphere and historical data are not available or adequate to determine the acute toxicity of the vehicle, a vehicle control group must be used. The vehicle control group must be a sham-treated group. Except for treatment with the test substance, animals in the vehicle control group must be handled in a manner identical to the test-group animals.
(E) Housing. The animals may be group-caged by sex, but the number of animals per cage must not interfere with clear observation of each animal. The biological properties of the test substance or toxic effects (e.g., morbidity, excitability) may indicate a need for individual caging. Animals must be housed individually in inhalation chambers during exposure to aerosols.
(1) Before and after exposure, the temperature of the animal room should be 22 ± 3 °C and the relative humidity 30-70%.
(2) Where lighting is artificial, the sequence should be 12 hours light/12 hours dark.
(3) For feeding, conventional laboratory diets may be used with an unlimited supply of drinking water.
(F) Inhalation equipment. (1) Animals can be exposed to the substance by either a nose-only procedure or in a whole-body exposure chamber. Maintenance of slight negative pressure inside the chamber will prevent leakage of the test substance into the surrounding areas. The nose-only exposure procedure is recommended for studies of aerosols to minimize exposures confounding resultant from test substance ingestion due to test animal fur licking following exposures. Animals must be acclimated to the nose-only exposure chamber prior to study and heat stress minimized during testing.
(2) Inhalation chambers. The animals must be tested in inhalation equipment designed to sustain a dynamic airflow for nose-only exposures of at least 300 ml/minute/animal or an airflow for whole-body exposures of at least 12 to 15 air changes per hour and ensure an adequate oxygen content of at least 19% and an evenly distributed exposure atmosphere. Where a whole-body chamber is used, its design must minimize crowding by providing individual caging. As a general rule, to ensure stability of a chamber atmosphere, the total “volume” of the test animals should not exceed 5% of the volume of the test chamber.
(3) Environmental conditions. The temperature at which the test is performed must be maintained at 22 °C (± 2 °C). Ideally, the relative humidity should be maintained between 40% and 60%, but in certain instances (e.g., tests using water as a vehicle), this may not be practical.
(G) Physical measurements. Measurements or monitoring must be made of the following:
(1) Chemical purity of the test material must be analyzed. If the test substance is present in a mixture, the mass and composition of the entire mixture, as well as the principal compound, must be measured. If there is some difficulty in measuring chamber analytical concentration due to precipitation, nonhomogeneous mixtures, volatile components, or other factors, additional analyses of components may be necessary.
(2) The rate of air flow should be monitored continuously, and must be recorded at least every 30 minutes during the exposure period.
(3) The actual concentrations of the test substance must be measured in the breathing zone. During the exposure period, the actual concentrations of the test substance must be held as constant as practicable, monitored continuously or intermittently depending on the method of analysis, and recorded at least three times (i.e., at the beginning, at an intermediate time, and at the end) during the exposure period. Chamber concentration may be measured using gravimetric or analytical methods as appropriate. If trial run measurements are reasonably consistent (± 10% for liquid aerosol, gas, or vapor; ± 20% for dry aerosol), then a minimum of two measurements are sufficient. If measurements are not consistent, then a minimum of four measurements should be taken.
(4) During the development of the generating system, particle size analysis must be performed to establish the stability of aerosol concentrations. During exposure, analysis should be conducted as often as necessary to determine the consistency of particle size distribution. The MMAD particle size range should be between 1-4 mm. The particle size of hygroscopic materials must be small enough when dry to assure that the size of the swollen particle will still be within the 1-4 mm MMAD range. Characterization for fibers must include the bivariate distribution of length and diameter; this distribution must ensure inhalability. Measurements of aerodynamic particle size in the animal's breathing zone must be measured during a trial run. If MMAD values for each exposure level are within 10% of each other, then a minimum of two measurements during the exposures should be sufficient. If pretest measurements are not within 10% of each other, then a minimum of four measurements should be taken.
(5) Temperature and humidity must be monitored continuously, and must be recorded at least every 30 minutes.
(iii) Exposure duration and concentration levels. (A) Exposure duration. Shortly before exposure, the animals are weighed and then exposed to the test target concentration in the designated apparatus for 4 hour exposure period after equilibration of the chamber concentrations. The target concentration is defined by an average of 5% for gases and vapors and 15% for particles and aerosols. The animals are weighed again at the conclusion of the exposure period to determine body weight change. Other durations may be needed to meet specific requirements. Food must be withheld during exposure. Water may also be withheld in certain circumstances.
(B) Exposure concentration levels. At least three concentration levels and a vehicle control group, if required (see paragraph (e)(3)(ii)(D)(2) of this section), must be used. The concentration levels should be spaced appropriately to produce a concentration-response curve and permit an estimation of the median lethal concentration (LC50). The concentrations can either be linearly or logarithmically spaced depending on the anticipated steepness of the concentration-response curve. A rationale for concentration selection should be provided to indicate that the selected concentrations will maximally support detection of concentration-Start Printed Page 78779response relationship. The high concentration should be clearly toxic or a limit concentration, but should not result in an incidence of fatalities that would preclude a meaningful evaluation of the data. The lowest concentration should define a no-observed-effects level (NOEL). Range-finding studies using single animals may help to estimate the positioning of the test groups so that no more than three concentration levels will be necessary.
(C) When the physical and chemical properties of the test substance show a low flash point or the test substance is otherwise known or thought to be explosive, care must be taken to avoid exposure level concentrations that could result in an exposure chamber explosion during the test.
(iv) Observation period. The observation period must be at least 14 days. However, the duration of observation should not be fixed rigidly. It should be determined by the toxic reactions, rate of onset, and length of recovery period, and thus may be extended when considered necessary. The time at which signs of toxicity appear, the duration of the signs observed, and the time of death must be recorded and are important, especially if there is a tendency for delayed effects.
(v) Observation of animals. (A) A careful clinical examination must be made at least once each day.
(B) Additional observations should be made daily with appropriate actions taken to minimize loss of animals to the study, e.g., necropsy or refrigeration of those animals found dead and isolation of weak or moribund animals.
(C) Observations must be detailed and carefully recorded, preferably using explicitly defined scales. Observations should include, but not be limited to, evaluation of skin and fur, eyes and mucous membranes, respiratory and circulatory effects, autonomic effects such as salivation, central nervous system effects, including tremors and convulsions, changes in the level of activity, gait and posture, reactivity to handling or sensory stimuli, altered strength, and stereotypies or bizarre behavior (e.g., self mutilation, walking backwards).
(D) Individual weights of animals must be determined pre-exposure and post-exposure, weekly after exposure, and at death. Changes in weights should be calculated and recorded when survival exceeds 1 day.
(E) The time of death should be recorded as precisely as possible.
(vi) Gross pathology. (A) At the end of the test, surviving animals must be weighed, sacrificed and a gross necropsy must be performed on all animals under test, with particular reference to any changes in the respiratory tract. All gross pathology changes must be recorded.
(1) The gross necropsy must include examination of orifices and the cranial, thoracic, and abdominal cavities, and contents.
(2) At least the lungs, liver, kidneys, adrenals, brain, and gonads should be weighed wet, as soon as possible after dissection to avoid drying.
(3) Optionally, the following organs and tissues, or representative samples thereof, may be preserved in a suitable medium for possible future histopathological examination: All gross lesions; brain-including sections of medulla/pons; cerebellar cortex and cerebral cortex; pituitary; thyroid/parathyroid; thymus; heart; sternum with bone marrow; salivary glands; liver; spleen; kidneys; adrenals; pancreas; gonads; accessory genital organs (epididymis, prostrate, and, if present, seminal vesicles); aorta; skin; gall bladder (if present); esophagus; stomach; duodenum; jejunum; ileum; cecum; colon; rectum; urinary bladder; representative lymph nodes; thigh musculature; peripheral nerve; spinal cord at three levels cervical, midthoracic, and lumbar; and eyes. Respiratory tract tissues should be perfusion preserved in a suitable medium.
(B) If necropsy cannot be performed immediately after a dead animal is discovered during the observation period, the animal should be refrigerated (not frozen) at temperatures low enough to minimize autolysis. Necropsies should be performed as soon as possible after death (normally within 24 to 48 hours).
(vii) Additional evaluations. In animals surviving 24 hours or more, microscopic examination of organs showing evidence of gross pathology should be considered since it may yield useful information on the nature of acute toxic effects.
(f) Data and reporting—(1) Treatment of results. Data must be summarized in tabular form showing for each test group the number of animals at the start of the test, body weights, time of death of individual animals at different exposure levels, number of animals displaying other signs of toxicity, description of toxic effects and necropsy findings. The method used for calculation of the LC50 or any other parameters must be specified and referenced. Some acceptable methods for parameter estimation are described in the references described in paragraphs (g)(1), (g)(2), and (g)(3) of this section.
(2) Evaluation of results. The LC50 value should be considered in conjunction with the observed toxic effects and the necropsy findings. The evaluation should include the relationship, if any, between exposure of animals to the test substance and the incidence and severity of all abnormalities including behavioral and clinical abnormalities, gross lesions, body weight changes, mortality, and other toxic effects.
(3) Test report. In addition to the reporting requirements specified under EPA Good Laboratory Practice Standards at 40 CFR part 792, subpart J, the following specific information must be reported. The test report shall include:
(i) Test conditions. (A) Description of exposure apparatus including design, type, dimensions.
(B) Source of air, system for generating the test article as particle, aerosol, gas, or vapor.
(C) Method for conditioning air, equipment for measuring temperature, humidity, particle size or particulate aerosol concentration size, and actual concentration.
(D) Treatment of exhaust air and the method of housing the animals in a test chamber when this is used.
(ii) Exposure data. The exposure data must be tabulated and presented with mean values and a measure of variability (e.g., standard deviation) and should include:
(A) Chemical purity of the test material.
(B) Airflow rates through the inhalation equipment.
(C) Temperature and humidity of the air.
(D) Nominal concentration (total amount of test substance fed into the inhalation equipment divided by volume of air).
(E) Actual (analytical or gravimetric) concentration in test breathing zone.
(F) Particle size distribution (calculated MMAD and GSD) and the bivariate distribution of fiber length and diameter, where appropriate.
(G) Explanation as to why the desired chamber concentration and/or particle size could not be achieved (if applicable), and the efforts taken to comply with these aspects of this section.
(iii) Species, strain, sex, and source of test animals.
(iv) Method of randomization in assigning animals to test and control groups.
(v) Rationale for selection of species, if other than that recommended.
(vi) Results. Tabulation of individual and test group data by sex and exposure Start Printed Page 78780concentration level (e.g., number of animals exposed, number of animals showing signs of toxicity and number of animals that died or were sacrificed during the test).
(A) Description of toxic effects including time of onset, duration, reversibility, and relationship to the exposure concentration levels.
(B) Pre-exposure and post-exposure body weight change in animals, and weight change during the observation period.
(C) Time of dosing and time of death during or following exposure.
(D) Concentration-response curves for mortality and other toxic effects (when permitted by the method of determination).
(E) Gross pathology necropsy findings in the test animals and vehicle control animals, if included. Data must be tabulated to show the counts and incidence of gross alterations observed for each group tested and the number of animals affected by each type of lesion along with the location and frequency of each type of lesion.
(F) Histopathology findings and any additional evaluations (e.g., clinical chemistry), if performed.
(vii) Description of any pretest conditioning, including diet, quarantine and treatment for disease.
(viii) Description of caging conditions, including: number (or change in number) of animals per cage, bedding material, ambient temperature and humidity, photoperiod, and identification of diet of test animals.
(ix) Manufacturer (source), lot number, and purity of test substance.
(x) Identification and composition of any vehicles (e.g., diluents, suspending agents, and emulsifiers) or other materials , if used in administering the test substance.
(xi) A list of references cited in the body of the report. References to any published literature used in developing the test protocol, performing the testing, making and interpreting observations, and compiling and evaluating the results.
(g) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., NW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Chanter, D.O. and Heywood, R. The LD50 test: some considerations of precision. Toxicology Letters 10:303 307 (1982).
(2) Finney, D.G. Chapter 3 Estimation of the median effective dose, Chapter 4 Maximum likelihood estimation. Probit Analysis. 3rd Ed. (Cambridge, London. (1971).
(3) Finney, D.J. The Median Lethal Dose and Its Estimation, Archives of Toxicology 56:215 218 (1985).
(4) Organization for Economic Cooperation and Development. OECD Guidelines for the Testing of Chemicals. Final Draft OECD Guideline 425: Acute Oral Toxicity: Up-and-Down Procedure to be adopted in the Tenth Addendum to the OECD Guidelines for the Testing of Chemicals.
(5) Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals. Guideline 403: Acute Inhalation Toxicity. Adopted: May 12, 1981.
(6) Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals. Guideline 420: Acute Oral Toxicity Fixed Dose Method. Adopted: July 17, 1992.
(7) Organization for Economic Cooperation and Development. OECD Guidelines for Testing of Chemicals. Guideline 423: Acute Oral Toxicity Acute Toxic Class Method. Adopted: March 22, 1996.
(8) U. S. EPA. Interim Policy for Particle Size and Limit Concentration Issues in Inhalation Toxicity Studies. 2/1/94. Health Effects Division, Office of Pesticide Programs.
TSCA Repeated dose 28-day oral toxicity study in rodents.(a) Scope—(1) Applicability. This section is intended to meet testing requirements of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides and Toxic Substances (OPPTS) harmonized test guideline 870.3050 (July 2000, final guidelines). This source is available at the address in paragraph (h) of this section.
(b) Purpose. (1) In the assessment and evaluation of the toxic characteristics of a chemical, the determination of oral toxicity using repeated doses may be carried out after initial information on toxicity has been obtained by acute testing. This study provides information on the possible health hazards likely to arise from repeated exposure over a relatively limited period of time. The method comprises the basic repeated dose toxicity study that may be used for chemicals on which a 90-day study is not warranted (e.g., when the production volume does not exceed certain limits) or as a preliminary to a long term study. The duration of exposure should normally be 28 days although a 14-day study may be appropriate in certain circumstances; justification for use of a 14-day exposure period should be provided.
(2) This section places emphasis on neurological effects as a specific endpoint, and the need for careful clinical observations of the animals, so as to obtain as much information as possible, is stressed. The method should identify chemicals with neurotoxic potential, which may warrant further in-depth investigation of this aspect. In addition, the method may give an indication of immunological effects and reproductive organ toxicity.
(c) Definitions. The definitions in section 3 of TSCA and in 40 CFR Part 792—Good Laboratory Practice Standards apply to this section. The following definitions also apply to this section.
Dosage is a general term comprising of dose, its frequency and the duration of dosing.
Dose is the amount of test substance administered. Dose is expressed as weight (g, mg) or as weight of test substance per unit weight of test animal (e.g., mg/kg), or as constant dietary concentrations (parts per million (ppm)).
No-observed-effects level (NOEL) is the maximum dose used in a study which produces no adverse effects. The NOEL is usually expressed in terms of the weight of a test substance given daily per unit weight of test animals (milligrams per kilograms per day).
(d) Principle of the test. The test substance is orally administered daily in graduated doses to several groups of experimental animals, one dose level per group for a period of 28 days. During the period of administration the animals are observed closely, each day for signs of toxicity. Animals which die or are sacrificed during the test are necropsied and at the conclusion of the test surviving animals are sacrificed and necropsied.
(e) Description of the method—(1) Selection of animal species. The preferred rodent species is the rat, although other rodent species may be used. Commonly used laboratory strains of young healthy adult animals should be employed. The females should be nulliparous and non-pregnant. Dosing should begin as soon as possible after weaning and, in any case, before the animals are 9 weeks old. At the commencement of the study the weight variation of animals used should be minimal and not exceed ± 20% of the mean weight of each sex. Where a repeated dose oral study is conducted as Start Printed Page 78781a preliminary to a long term study, preferably animals from the same strain and source should be used in both studies.
(2) Housing and feeding conditions. The temperature in the experimental animal room should be 22 °C (± 3 °C). Although the relative humidity should be at least 30% and preferably not to exceed 70% other than during room cleaning, the aim should be 50-60%. Lighting should be artificial, the sequence being 12 hours light, 12 hours dark. For feeding, conventional laboratory diets may be used with an unlimited supply of drinking water. The choice of diet may be influenced by the need to ensure a suitable admixture of a test substance when administered by this method. Animals may be housed individually, or be caged in small groups of the same sex; for group caging, no more than five animals should be housed per cage.
(3) Preparation of animals. Healthy young adult animals must be randomly assigned to the control and treatment groups. Cages should be arranged in such a way that possible effects due to cage placement are minimized. The animals are identified uniquely and kept in their cages for at least 5 days prior to the start of the study to allow for acclimatization to the laboratory conditions.
(4) Preparation of doses. (i) The test compound must be administered by gavage or via the diet or drinking water. The method of oral administration is dependent on the purpose of the study, and the physical/chemical properties of the test material.
(ii) Where necessary, the test substance is dissolved or suspended in a suitable vehicle. It is recommended that, wherever possible, the use of an aqueous solution/suspension be considered first, followed by consideration of a solution/emulsion in oil (e.g., corn oil) and then by possible solution in other vehicles. For vehicles other than water the toxic characteristics of the vehicle must be known. The stability of the test substance in the vehicle should be determined.
(f) Procedure—(1) Number and sex of animals. At least 10 animals (five female and five male) should be used at each dose level. If interim sacrifices are planned, the number should be increased by the number of animals scheduled to be sacrificed before the completion of the study. Consideration should be given to an additional satellite group of 10 animals (five per sex) in the control and in the top dose group for observation of reversibility, persistence, or delayed occurrence of toxic effects, for at least 14 days post treatment.
(2) Dosage. (i) Generally, at least three test groups and a control group should be used, but if from assessment of other data, no effects would be expected at a dose of 1000 mg/kg bodyweight/per day, a limit test may be performed. If there are no suitable data available, a range finding study may be performed to aid the determination of the doses to be used. Except for treatment with the test substance, animals in the control group should be handled in an identical manner to the test group subjects. If a vehicle is used in administering the test substance, the control group should receive the vehicle in the highest volume used.
(ii) Dose levels should be selected taking into account any existing toxicity and (toxico-) kinetic data available for the test compound or related materials. The highest dose level should be chosen with the aim of inducing toxic effects but not death or severe suffering. Thereafter, a descending sequence of dose levels should be selected with a view to demonstrating any dosage related response and NOEL at the lowest dose level. Two to four fold intervals are frequently optimal for setting the descending dose levels and addition of a fourth test group is often preferable to using very large intervals (e.g., more than a factor of 10) between dosages.
(3) Limit test. If a test at one dose level of at least 1000 mg/kg body weight/day or, for dietary or drinking water administration, an equivalent percentage in the diet, or drinking water (based upon body weight determinations), using the procedures described for this study, produces no observable toxic effects and if toxicity would not be expected based upon data from structurally related compounds, then a full study using three dose levels may not be considered necessary. The limit test applies except when human exposure indicates the need for a higher dose level to be used.
(4) Administration of doses. (i) The animals are dosed with the test substance daily 7 days each week for a period of 28 days; use of a 5-day per week dosing regime or a 14-day exposure period needs to be justified. When the test substance is administered by gavage, this should be done in a single dose to the animals using a stomach tube or a suitable intubation cannula. The maximum volume of liquid that can be administered at one time depends on the size of the test animal. The volume should not exceed 1ml/100g body weight, except in the case of aqueous solutions where 2ml/100g body weight may be used. Except for irritating or corrosive substances which will normally reveal exacerbated effects with higher concentrations, variability in test volume should be minimized by adjusting the concentration to ensure a constant volume at all dose levels.
(ii) For substances administered via the diet or drinking water it is important to ensure that the quantities of the test substance involved do not interfere with normal nutrition or water balance. When the test substance is administered in the diet either a constant dietary concentration (parts per million (ppm)) or a constant dose level in terms of the animals' body weight may be used; the alternative used must be specified. For a substance administered by gavage, the dose should be given at similar times each day, and adjusted as necessary to maintain a constant dose level in terms of animal body weight. Where a repeated dose study is used as a preliminary to a long term study, a similar diet should be used in both studies.
(5) Observations. (i) The observation period should be 28 days, unless the study duration is 14 days (see paragraph (b)(1) of this section). Animals in a satellite group scheduled for follow-up observations should be kept for at least a further 14 days without treatment to detect delayed occurrence, or persistence of, or recovery from toxic effects.
(ii) General clinical observations should be made at least once a day, preferably at the same time(s) each day and considering the peak period of anticipated effects after dosing. The health condition of the animals should be recorded. At least twice daily, all animals are observed for morbidity and mortality.
(iii) Once before the first exposure (to allow for within-subject comparisons), and at least once a week thereafter, detailed clinical observations should be made in all animals. These observations should be made outside the home cage in a standard arena and preferably at the same time, each time. They should be carefully recorded, preferably using scoring systems, explicitly defined by the testing laboratory. Effort should be made to ensure that variations in the test conditions are minimal and that observations are preferably conducted by observers unaware of the treatment. Signs noted should include, but not be limited to, changes in skin, fur, eyes, mucous membranes, occurrence of secretions and excretions and autonomic activity (e.g., lacrimation, piloerection, pupil size, unusual respiratory pattern). Changes in gait, posture and response to handling as Start Printed Page 78782well as the presence of clonic or tonic movements, stereotypies (e.g., excessive grooming, repetitive circling) or bizarre behaviour (e.g., self-mutilation, walking backwards) should also be recorded.
(iv) In the fourth exposure week sensory reactivity to stimuli of different types (see paragraph (h)(2) of this section) (e.g., auditory, visual and proprioceptive stimuli), assessment of grip strength and motor activity assessment should be conducted. Further details of the procedures that could be followed are given in the respective references. However, alternative procedures than those referenced could also be used. Examples of procedures for observation are described in the references in paragraphs (h)(1), (h)(2), (h)(3), (h)(4), and (h)(5) of this section.
(v) Functional observations conducted in the fourth exposure week may be omitted when the study is conducted as a preliminary study to a subsequent subchronic (90-day) study. In that case, the functional observations should be included in this follow-up study. On the other hand, the availability of data on functional observations from the repeated dose study may enhance the ability to select dose levels for a subsequent subchronic study.
(vi) Exceptionally, functional observations may also be omitted for groups that otherwise reveal signs of toxicity to an extent that would significantly interfere with the functional test performance.
(6) Body weight and food/water consumption. All animals should be weighed at least once a week. Measurements of food consumption should be made at least weekly. If the test substance is administered via the drinking water, water consumption should also be measured at least weekly.
(7) Hematology. (i) The following hematological examinations should be made at the end of the test period: hematocrit, hemoglobin concentration, erythrocyte count, total and differential leukocyte count, platelet count and a measure of blood clotting time/potential.
(ii) Blood samples should be taken from a named site just prior to or as part of the procedure for sacrificing the animals, and stored under appropriate conditions.
(8) Clinical Biochemistry. (i) Clinical biochemistry determinations to investigate major toxic effects in tissues and, specifically, effects on kidney and liver, should be performed on blood samples obtained of all animals just prior to or as part of the procedure for sacrificing the animals (apart from those found moribund and/or intercurrently sacrificed). Overnight fasting of the animals prior to blood sampling is recommended.[1] Investigations of plasma or serum shall include sodium, potassium, glucose, total cholesterol, urea, creatinine, total protein and albumin, at least two enzymes indicative of hepatocellular effects (such as alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma glutamyl transpeptidase, and sorbitol dehydrogenase). Measurements of additional enzymes (of hepatic or other origin) and bile acids may provide useful information under certain circumstances.
(ii) Optionally, the following urinalysis determinations could be performed during the last week of the study using timed urine volume collection; appearance, volume, osmolality or specific gravity, pH, protein, glucose and blood and blood cells.
(iii) In addition, studies to investigate serum markers of general tissue damage should be considered. Other determinations that should be carried out if the known properties of the test substance may, or are suspected to, affect related metabolic profiles include calcium, phosphate, fasting triglycerides, specific hormones, methemoglobin and cholinesterase. These must to be identified for chemicals in certain classes or on a case-by-case basis.
(iv) Overall, there is a need for a flexible approach, depending on the species and the observed and/or expected effect with a given compound.
(v) If historical baseline data are inadequate, consideration should be given to determination of hematological and clinical biochemistry variables before dosing commences.
(9) Pathology—(i) Gross necropsy. (A) All animals in the study must be subjected to a full, detailed gross necropsy which includes careful examination of the external surface of the body, all orifices, and the cranial, thoracic and abdominal cavities and their contents. The liver, kidneys, adrenals, testes, epididymides, thymus, spleen, brain and heart of all animals (apart from those found moribund and/or intercurrently sacrificed) should be trimmed of any adherent tissue, as appropriate, and their wet weight taken as soon as possible after dissection to avoid drying.
(B) The following tissues should be preserved in the most appropriate fixation medium for both the type of tissue and the intended subsequent histopathological examination: all gross lesions, brain (representative regions including cerebrum, cerebellum and pons), spinal cord, stomach, small and large intestines (including Peyer's patches), liver, kidneys, adrenals, spleen, heart, thymus, thyroid, trachea and lungs (preserved by inflation with fixative and then immersion), ovaries, uterus, testes, epididymides, accessory sex organs (e.g., prostate, seminal vesicles), urinary bladder, lymph nodes (preferably one lymph node covering the route of administration and another one distant from the route of administration to cover systemic effects), peripheral nerve (sciatic or tibial) preferably in close proximity to the muscle, and a section of bone marrow (or, alternatively, a fresh mounted bone marrow aspirate). The clinical and other findings may suggest the need to examine additional tissues. Also any organs considered likely to be target organs based on the known properties of the test substance should be preserved.
(ii) Histopathology. (A) Full histopathology should be carried out on the preserved organs and tissues of all animals in the control and high dose groups. These examinations should be extended to animals of all other dosage groups, if treatment-related changes are observed in the high dose group.
(B) All gross lesions must be examined.
(C) When a satellite group is used, histopathology should be performed on tissues and organs identified as showing effects in the treated groups.
(g) Data and reporting—(1) Data. (i) Individual data should be provided. Additionally, all data should be summarized in tabular form showing for each test group the number of animals at the start of the test, the number of animals found dead during the test or sacrificed for humane reasons and the time of any death or humane sacrifice, the number showing signs of toxicity, a description of the signs of toxicity observed, including time of onset, duration, and severity of any toxic effects, the number of animals showing lesions, the type of lesions and the percentage of animals displaying each type of lesion. Start Printed Page 78783
(ii) When possible, numerical results should be evaluated by an appropriate and generally acceptable statistical method. The statistical methods should be selected during the design of the study.
(2) Test report. The test report must include the following information:
(i) Test substance:
(A) Physical nature, purity and physicochemical properties.
(B) Identification data.
(ii) Vehicle (if appropriate): Justification for choice of vehicle, if other than water.
(iii) Test animals:
(A) Species/strain used.
(B) Number, age and sex of animals.
(C) Source, housing conditions, diet, etc.
(D) Individual weights of animals at the start of the test.
(iv) Test conditions:
(A) Rationale for dose level selection.
(B) Details of test substance formulation/diet preparation, achieved concentration, stability and homogeneity of the preparation.
(C) Details of the administration of the test substance.
(D) Conversion from diet/drinking water test substance concentration (parts per million (ppm)) to the actual dose (mg/kg body weight/day), if applicable.
(E) Details of food and water quality.
(v) Results:
(A) Body weight/body weight changes.
(B) Food consumption, and water consumption, if applicable.
(C) Toxic response data by sex and dose level, including signs of toxicity.
(D) Nature, severity and duration of clinical observations (whether reversible or not).
(E) Sensory activity, grip strength and motor activity assessments.
(F) Hematological tests with relevant base-line values.
(G) Clinical biochemistry tests with relevant base-line values.
(H) Body weight at sacrificing and organ weight data.
(I) Necropsy findings.
(J) A detailed description of all histopathological findings.
(K) Absorption data if available.
(L) Statistical treatment of results, where appropriate.
(vi) Discussion of results.
(vii) Conclusions.
(h) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., SW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Tupper, D.E., Wallace, R.B. (1980). Utility of the Neurologic Examination in Rats. Acta Neurobiological Exposure, 40:999-1003.
(2) Gad, S.C. (1982). A Neuromuscular Screen for Use in Industrial Toxicology. Journal of Toxicology and Environmental Health, 9:691-704.
(3) Moser, V.C., McDaniel, K.M., Phillips, P.M. (1991). Rat Strain and Stock Comparisons Using a Functional Observational Battery: Baseline Values and Effects of Amitraz. Toxicology and Applied Pharmacology, 108:267-283.
(4) Meyer O.A., Tilson H.A., Byrd W.C., Riley M.T. (1979). A Method forthe Routine Assessment of Fore- and Hindlimb Grip Strength of Rats and Mice. Neurobehavioral Toxicology, 1:233-236.
(5) Crofton K.M., Howard J.L., Moser V.C., Gill M.W., Reiter L.W., Tilson H.A., MacPhail R.C. (1991). Interlaboratory Comparison of Motor Activity Experiments: Implication for Neurotoxicological Assessments. Neurotoxicology and Teratology, 13:599-609.
TSCA 90-day oral toxicity in rodents.(a) Scope. This section is intended to meet the testing requirements under section 4 of the Toxic Substances Control Act (TSCA). In the assessment and evaluation of the toxic characteristics of a chemical, the determination of subchronic oral toxicity may be carried out after initial information on toxicity has been obtained by acute testing. The subchronic oral study has been designed to permit the determination of the no-observed-effects level (NOEL) and toxic effects associated with continuous or repeated exposure to a test substance for a period of 90 days. This study is not capable of determining those effects that have a long latency period for development (e.g., carcinogenicity and life shortening). Extrapolation from the results of this study to humans is valid only to a limited degree. However, it can useful in providing information on health hazards likely to arise from repeated exposure by the oral route over a limited period of time, such as target organs, the possibilities of accumulation, and can be of use in selecting dose levels for chronic studies and for establishing safety criteria for human exposure.
(b) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides, and Toxic Substances (OPPTS) harmonized test guideline 870.3100 (August 1998, final guideline). This source is available at the address in paragraph (h) of this section.
(c) Definitions. The following definitions apply to this section.
Cumulative toxicity is the adverse effects of repeated doses occurring as a result of prolonged action on, or increased concentration of, the administered test substance or its metabolites in susceptible tissue.
Dose in a subchronic oral study is the amount of test substance administered daily via the oral route (gavage, drinking water or diet) for a period of 90 days. Dose is expressed as weight of the test substance (grams, milligrams) per unit body weight of test animal (milligram per kilogram) or as weight of the test substance in parts per million in food or drinking water per day.
No-observed-effects level (NOEL) is the maximum dose used in a study which produces no adverse effects. The NOEL is usually expressed in terms of the weight of a test substance given daily per unit weight of test animal (milligrams per kilogram per day).
Subchronic oral toxicity is the adverse effects occurring as a result of the repeated daily exposure of experimental animals to a chemical by the oral route for a part (approximately 10%) of the test animal's life span.
Target organ is any organ of a test animal showing evidence of an effect induced by a test substance.
(d) Limit test. If a test at one dose level of at least 1,000 mg/kg body weight (expected human exposure may indicate the need for a higher dose level), using the procedures described for this study, produces no observable toxic effects or if toxic effects would not be expected based upon data of structurally related compounds, then a full study using three dose levels might not be necessary.
(e) Test procedures—(1) Animal selection—(i) Species and strain. A variety of rodent species may be used, although the rat is the preferred species. Commonly used laboratory strains must be employed.
(ii) Age/weight. (A) Testing should be started with young healthy animals as soon as possible after weaning and acclimatization.
(B) Dosing of rodents should generally begin no later than 8-9 weeks of age.
(C) At the commencement of the study the weight variation of animals used must be within 20% of the mean weight for each sex.
(iii) Sex. Equal numbers of animals of each sex must be used at each dose Start Printed Page 78784level, and the females shall be nulliparous and nonpregnant.
(iv) Numbers. (A) At least 20 rodents (10 males and 10 females) at each dose level.
(B) If interim sacrifices are planned, the number must be increased by the number of animals scheduled to be sacrificed before the completion of the study.
(C) To avoid bias, the use of adequate randomization procedures for the proper allocation of animals to test and control groups is required.
(D) Each animal must be assigned a unique identification number. Dead animals, their preserved organs and tissues, and microscopic slides must be identified by reference to the animal's unique number.
(v) Husbandry. (A) Animals may be group-caged by sex, but the number of animals per cage must not interfere with clear observation of each animal. The biological properties of the test substance or toxic effects (e.g., morbidity, excitability) may indicate a need for individual caging.
(B) The temperature of the experimental animal rooms should be at 22 ± 3 °C.
(C) The relative humidity of the experimental animal rooms should be 50 ± 20%.
(D) Where lighting is artificial, the sequence should be 12 hours light/12 hours dark.
(E) Control and test animals must be fed from the same batch and lot. The feed should be analyzed to assure adequacy of nutritional requirements of the species tested and for impurities that might influence the outcome of the test. For feeding, conventional laboratory diets may be used with an unlimited supply of drinking water.
(F) The study should not be initiated until animals have been allowed a period of acclimatization/quarantine to environmental conditions, nor should animals from outside sources be placed on test without an adequate period of quarantine. An acclimation period of at least five days is recommended.
(2) Control and test substances. (i) Where necessary, the test substance is dissolved or suspended in a suitable vehicle. If a vehicle or diluent is needed, the vehicle should not elicit toxic effects or substantially alter the chemical or toxicological properties of the test substance. It is recommended that wherever possible the usage of an aqueous solution be considered first, followed by consideration of a solution in oil and then solution in other vehicles.
(ii) If possible, one lot of the test substance tested should be used throughout the duration of the study and the research sample should be stored under conditions that maintain its purity and stability. Prior to the initiation of the study, there should be a characterization of the test substance, including the purity of the test compound and, if technically feasible, the names and quantities of contaminants and impurities.
(iii) If the test or control substance is to be incorporated into feed or another vehicle, the period during which the test substance is stable in such a mixture should be determined prior to the initiation of the study. Its homogeneity and concentration should be determined prior to the initiation of the study and periodically during the study. Statistically randomized samples of the mixture should be analyzed to ensure that proper mixing, formulation, and storage procedures are being followed, and that the appropriate concentration of the test or control substance is contained in the mixture.
(3) Control groups. A concurrent control group is required. This group must be an untreated or sham-treated control group or, if a vehicle is used in administering the test substance, a vehicle control group. If the toxic properties of the vehicle are not known or cannot be made available, both untreated and vehicle control groups are required.
(4) Satellite group. A satellite group of 20 animals (10 animals per sex) may be treated with the high dose level for 90 days and observed for reversibility, persistence, or delayed occurrence of toxic effects for a post-treatment period of appropriate length, normally not less than 28 days. In addition, a control group of 20 animals (10 animals of each sex) should be added to the satellite study.
(5) Dose levels and dose selection. (i) In subchronic toxicity tests, it is desirable to determine a dose-response relationship as well as a NOEL. Therefore, at least three dose levels plus a control and, where appropriate, a vehicle control (corresponding to the concentration of vehicle at the highest dose level) must be used. Doses should be spaced appropriately to produce test groups with a range of toxic effects. The data should be sufficient to produce a dose-response curve.
(ii) The highest dose level should result in toxic effects but not produce an incidence of fatalities which would prevent a meaningful evaluation.
(iii) The intermediate dose levels should be spaced to produce a gradation of toxic effects.
(iv) The lowest dose level should produce no evidence of toxicity.
(6) Administration of the test substance. (i) If the test substance is administered by gavage, the animals are dosed with the test substance on a 7-day per week basis for a period of at least 90 days. However, based primarily on practical considerations, dosing by gavage on a 5-day per week basis is acceptable. If the test substance is administered in the drinking water, or mixed in the diet, then exposure should be on a 7-day per week basis.
(ii) All animals must be dosed by the same method during the entire experimental period.
(iii) For substances of low toxicity, it is important to ensure that when administered in the diet the quantities of the test substance involved do not interfere with normal nutrition. When the test substance is administered in the diet, either a constant dietary concentration (parts per million) or a constant dose level in terms of body weight should be used; the alternative used should be specified.
(iv) For a substance administered by gavage, the dose should be given at approximately the same time each day, and adjusted at intervals (weekly or biweekly) to maintain a constant dose level in terms of body weight.
(7) Observation period. (i) The animals must be observed for a period of 90 days.
(ii) Animals in the satellite group (if used) scheduled for follow-up observations should be kept for at least 28 days further without treatment to detect recovery from, or persistence of, toxic effects.
(8) Observation of animals. (i) Observations must be made at least twice each day for morbidity and mortality. Appropriate actions should be taken to minimize loss of animals to the study (e.g., necropsy or refrigeration of those animals found dead and isolation or sacrifice of weak or moribund animals). General clinical observations should be made at least once a day, preferably at the same time each day, taking into consideration the peak period of anticipated effects after dosing. The clinical condition of the animal should be recorded.
(ii) A careful clinical examination must be made at least once weekly. Observations should be detailed and carefully recorded, preferably using explicity defined scales. Observations should include, but not be limited to, evaluation of skin and fur, eyes and mucous membranes, respiratory and circulatory effects, autonomic effects such as salivation, central nervous system effects, including tremors and convulsions, changes in the level of activity, gait and posture, reactivity to Start Printed Page 78785handling or sensory stimuli, altered strength, and stereotypes or bizarre behavior (e.g., self-mutilation, walking backwards).
(iii) Signs of toxicity should be recorded as they are observed including the time of onset, degree and duration.
(iv) Measurements of food consumption and water consumption, if drinking water is the exposure route, must be made weekly.
(v) Individual weights of animals must be determined shortly before the test substance is administered, weekly thereafter, and at death.
(vi) Moribund animals should be removed and sacrificed when noticed and the time of death should be recorded as precisely as possible.
(vii) At termination, all survivors in the treatment and control groups must be sacrificed.
(9) Clinical pathology. Hematology and clinical chemistry examinations must be made on all animals, including controls, of each sex in each group. The hematology and clinical chemistry parameters should be examined at terminal sacrifice at the end of the study. Overnight fasting of the animals prior to blood sampling is recommended. Overall, there is a need for a flexible approach in the measures examined, depending on the observed or expected effects from a chemical, and in the frequency of measures, depending on the duration of potential chemical exposures.
(i) Hematology. The recommended parameters are red blood cell count, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration, white blood cell count, differential leukocyte count, platelet count, and a measure of clotting potential, such as prothrombin time or activated partial thromboplastin time.
(ii) Clinical chemistry. (A) Parameters which are considered appropriate to all studies are electrolyte balance, carbohydrate metabolism, and liver and kidney function. The selection of specific tests will be influenced by observations on the mode of action of the substance and signs of clinical toxicity.
(B) The recommended clinical chemistry determinations are potassium, sodium, glucose, total cholesterol, urea nitrogen, creatinine, total protein and albumin. More than 2 hepatic enzymes, (such as alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, sorbitol dehydrogenase, or gamma glutamyl transpeptidase) should also be measured. Measurements of addtional enzymes (of hepatic or other origin) and bile acids, may also be useful.
(C) If a test chemical has an effect on the hematopoietic system, reticulocyte counts and bone marrow cytology may be indicated.
(D) Other determinations that should be carried out if the test chemical is known or suspected of affecting related measures include calcium, phosphorus, fasting triglycerides, hormones, methemoglobin, and cholinesterases.
(iii) Optionally, the following urinalysis determinations could be performed during the last week of the study using timed urine volume collection: appearance, volume, osmolality or specific gravity, pH, protein, glucose and blood/blood cells.
(10) Ophthalmological examination. Ophthalmological examinations using an ophthalmoscope or an equivalent device must be made on all animals prior to the administration of the test substance and on all high dose and control groups at termination. If changes in the eyes are detected, all animals in the other dose groups must be examined.
(11) Gross necropsy. (i) All animals must be subjected to a full gross necropsy which includes examination of the external surface of the body, all orifices, and the cranial, thoracic and abdominal cavities and their contents.
(ii) The liver, kidneys, adrenals, testes, epididymides, ovaries, uterus, thymus, spleen, brain, and heart must be trimmed and weighed wet, as soon as possible after dissection.
(iii) The following organs and tissues, or representative samples thereof, should be preserved in a suitable medium for possible future histopathological examination:
(A) Digestive system—salivary glands, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, liver, pancreas, gallbladder (when present).
(B) Nervous system—brain (including sections of medulla/pons, cerebellum and cerebrum), pituitary, peripheral nerve (sciatic or tibial, preferably in close proximity to the muscle), spinal cord (three levels: cervical, mid-thoracic and lumbar), eyes (retina, optic nerve).
(C) Glandular system—adrenals, parathyroid, thyroid.
(D) Respiratory system—trachea, lungs, pharynx, larynx, nose.
(E) Cardiovascular/hemopoietic system—aorta, heart, bone marrow (and/or fresh aspirate), lymph nodes (preferably one lymph node covering the route of administration and another one distant from the route of administration to cover systemic effects), spleen, thymus.
(F) Urogenital system—kidneys, urinary bladder, prostate, testes, epididymides, seminal vesicle(s), uterus, ovaries, female mammary gland.
(G) Others—all gross lesions and masses, skin.
(12) Histopathology. (i) The following histopathology must be performed:
(A) Full histopathology on the organs and tissues, listed in paragraph (e)(11)(iii) of this section, of all rodents in the control and high dose groups, and all rodents that died or were sacrificed during the study.
(B) All gross lesions in all animals.
(C) Target tissues in all animals.
(D) When a satellite group is used, histopathology should be performed on tissues and organs identified as showing effects in the treated groups.
(ii) If excessive early deaths or other problems occur in the high dose group compromising the significance of the data, the next dose level should be examined for complete histopathology.
(iii) An attempt should be made to correlate gross observations with microscopic findings.
(iv) Tissues and organs designated for microscopic examination should be fixed in 10% buffered formalin or a recognized suitable fixative as soon as necropsy is performed and no less than 48 hours prior to trimming.
(f) Data and reporting—(1) Treatment of results. (i) Data must be summarized in tabular form, showing for each test group the number of animals at the start of the test, the number of animals showing lesions, the types of lesions and the percentage of animals displaying each type of lesion.
(ii) When applicable, all observed results, qualitative and quantitative, should be evaluated by an appropriate and generally accepted statistical method. Any generally accepted statistical methods may be used; the statistical methods, including significance criteria, should be selected during the design of the study.
(2) Evaluation of study results. The findings of a subchronic oral toxicity study should be evaluated in conjunction with the findings of preceding studies and considered in terms of the toxic effects and the necropsy and histopathological findings. The evaluation must include the relationship between the dose of the test substance and the presence or absence, the incidence and severity, of abnormalities, including behavioral and clinical abnormalities, gross lesions, identified target organs, body weight changes, effects on mortality and any other general or specific toxic effects. A properly conducted subchronic test should provide a satisfactory estimation Start Printed Page 78786of a NOEL. It also can indicate the need for an additional longer-term study and provide information on the selection of dose levels.
(3) Test report. In addition to reporting requirements specified under EPA Good Laboratory Practice Standards at 40 CFR part 792, subpart J, the following specific information must be reported:
(i) Test substance characterization should include:
(A) Chemical identification.
(B) Lot or batch number.
(C) Physical properties.
(D) Purity/impurities.
(ii) Identification and composition of any vehicle used.
(iii) Test system should contain data on:
(A) Species and strain of animals used and rationale for selection if other than that recommended.
(B) Age including body weight data and sex.
(C) Test environment including cage conditions, ambient temperature, humidity, and light/dark periods.
(D) Identification of animal diet.
(E) Acclimation period.
(iv) Test procedure should include the following data:
(A) Method of randomization used.
(B) Full description of experimental design and procedure.
(C) Dose regimen including levels, methods, and volume.
(v) Test results should include:
(A) Group animal data. Tabulation of toxic response data by species, strain, sex and exposure level for:
(1) Number of animals exposed.
(2) Number of animals showing signs of toxicity.
(3) Number of animals dying.
(B) Individual animal data. Data should be presented as summary (group mean) as well as for individual animals.
(1) Date of death during the study or whether animals survived to termination.
(2) Date of observation of each abnormal sign and its subsequent course.
(3) Body weight data.
(4) Feed and water (if collected) consumption data.
(5) Achieved dose (mg/kg/day) as a time-weighted average if the test substance is administered in the diet or drinking water.
(6) Results of ophthalmological examination.
(7) Results of hematological tests performed.
(8) Results of clinical chemistry tests performed.
(9) Results of urinalysis, if performed.
(10) Necropsy findings, including absolute and relative (to body weight) organ weight data.
(11) Detailed description of all histopathological findings.
(12) Statistical treatment of results, where appropriate.
(g) Quality control. A system must be developed and maintained to assure and document adequate performance of laboratory equipment. The study must be conducted in compliance with 40 CFR Part 792—Good Laboratory Practice Standards.
(h) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., NW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Boyd, E.M. Chapter 14. Pilot Studies, 15. Uniposal Clinical Parameters, 16. Uniposal Autopsy Parameters. Predictive Toxicometrics. Williams and Wilkins, Baltimore (1972).
(2) Fitzhugh, O.G. Subacute Toxicity, Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics. The Association of Food and Drug Officials of the United States (1959, 3rd Printing 1975) pp. 26-35.
(3) Organization for Economic Cooperation and Development. OECD uidelines for Testing of Chemicals. Guideline 408: Subchronic Oral Toxicity-Rodent: 90-day Study, Adopted: May 12, 1981.
(4) Weingand K., Brown G., Hall R. et al. Harmonization of Animal Clinical Pathology Testing in Toxicity and Safety Studies. Fundam. & Appl. Toxicol. 29:198-201. (1996)
TSCA 90-day dermal toxicity.(a) Scope. This section is intended to meet the testing requirements under section 4 of the Toxic Substances Control Act (TSCA). In the assessment and evaluation of the toxic characteristics of a chemical, the determination of subchronic dermal toxicity may be carried out after initial information on toxicity has been obtained by acute testing. The subchronic dermal study has been designed to permit the determination of the no-observed-effects level (NOEL) and toxic effects associated with continuous or repeated exposure to a test substance for a period of 90 days. This study is not capable of determining those effects that have a long latency period for development (e.g., carcinogenicity and life shortening). Extrapolation from the results of this study to humans is valid only to a limited degree. It can, however, provide useful information on the degree of percutaneous absorption, target organs, the possibilities of accumulation, and can be of use in selecting dose levels for chronic studies and for establishing safety criteria for human exposure.
(b) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides, and Toxic Substances (OPPTS) harmonized test guideline 870.3250 (August 1998, final guideline). This source is available at the address in paragraph (h) of this section.
(c) Definitions. The following definitions also apply to this section.
Cumulative toxicity is the adverse effect of repeated doses occurring as a result of prolonged action or increased concentration of the administered test substance or its metabolites in susceptible tissues.
Dose in a subchronic dermal study is the amount of test substance applied daily to the skin for 90 days. Dose is expressed as weight of the test substance (grams, milligrams), per unit body weight of test animal (milligrams per kilogram), or as weight of the test substance per unit of surface area (milligrams per square centimeter) per day.
No-observed-effects level (NOEL) is the maximum dose used in a study which produces no adverse effects. The NOEL is expressed in terms of the weight of a test substance given daily per unit weight of test animal (milligrams per kilogram per day).
Subchronic dermal toxicity is the adverse effects occurring as a result of the repeated daily exposure of experimental animals to a chemical by the dermal route for a part of the test animal's life span.
Target organ is any organ of a test animal showing evidence of an effect induced by a test substance.
(d) Limit test. If a test at one dose level of at least 1,000 mg/kg body weight (expected human exposure may indicate the need for a higher dose level), using the procedures described for this section, produces no observable toxic effects or if toxic effects would not be expected based upon data on structurally related compounds, a full study using three dose levels might not be necessary.
(e) Test procedures—(1) Animal selection—(i) Species and strain. A mammalian species must be used for testing. The rat, rabbit, or guinea pig may be used. Commonly used laboratory strains must be employed. If other mammalian species are used, the tester must provide justification/reasoning for their selection. When a subchronic dermal study is conducted Start Printed Page 78787as a preliminary to a chronic dermal study, the same species and strain must be used in both studies.
(ii) Age/weight. (A) Testing should be started with young healthy animals as soon as possible after weaning and acclimatization.
(B) Dosing should generally begin in guinea pigs between 5-6 weeks of age, in rats between 8-9 weeks of age, and in rabbits at least 12 weeks old.
(C) At the commencement of the study, the weight variation of animals used must be within 20% of the mean weight for each sex.
(iii) Sex. Equal numbers of animals of each sex with healthy skin must be used at each dose level. The females shall be nulliparous and nonpregnant except for specially designed studies.
(iv) Numbers. (A) At least 20 animals (10 animals per sex) must be used at each dose level.
(B) If interim sacrifices are planned, the number must be increased by the number of animals scheduled to be sacrificed before completion of the study.
(C) To avoid bias, the use of adequate randomization procedures for the proper allocation of animals to test and control groups is required.
(D) Each animal must be assigned a unique identification number. Dead animals, their preserved organs and tissues, and microscopic slides must be identified by reference to the animal's unique number.
(v) Husbandry. (A) Animals should be housed in individual cages.
(B) The temperature of the experimental animal rooms should be at 22 ± 3 °C
(C) The relative humidity of the experimental animal rooms should be 50 ± 20%.
(D) Where lighting is artificial, the sequence should be 12 hours light/12 hours dark.
(E) Control and test animals must be fed from the same batch and lot. The feed should be analyzed to assure adequacy of nutritional requirements of the species tested and for impurities that might influence the outcome of the test. For feeding, conventional laboratory diets may be used with an unlimited supply of drinking water.
(F) The study should not be initiated until animals have been allowed a period of acclimatization/quarantine to environmental conditions, nor should animals from outside sources be placed on test without an adequate period of quarantine. An acclimation period of at least five days is recommended.
(2) Control and test substances. (i) Where necessary, the test substance is dissolved or suspended in a suitable vehicle. If a vehicle or diluent is needed, the vehicle should not elicit toxic effects or substantially alter the chemical or toxicological properties of the test substance. It is recommended that, whenever possible, the usage of an aqueous solution be considered first, followed by consideration of a solution of oil and then solution of other vehicles.
(ii) One lot of the test substance should be used, if possible, throughout the duration of the study, and the research sample should be stored under conditions that maintain its purity and stability. Prior to the initiation of the study, there should be a characterization of the test substance, including the purity of the test compound and if technically feasible, the name and quantities of unknown contaminants and impurities.
(iii) If the test substance is dissolved or suspended in a vehicle, the period during which the test substance is stable in such a mixture should be determined prior to the initiation of the study. Its homogeneity and concentration should be determined prior to the initiation of the study and periodically during the study. Statistically randomized samples of the mixture should be analyzed to ensure that proper mixing, formulation, and storage procedures are being followed, and that the appropriate concentration of the test or control substance is contained in the mixture.
(3) Control groups. A concurrent control group is required. This group must be an untreated or sham-treated control group or, if a vehicle is used in the application of the test substance, a vehicle control group. If the toxic properties of the vehicle are not known or not available, both untreated/sham-treated and vehicle control groups are required.
(4) Satellite group. A satellite group of 20 animals (10 animals per sex) may be treated with the high dose level for 90 days and observed for reversibility, persistence, or delayed occurrence of toxic effects for a post-treatment period of appropriate length, normally not less than 28 days. In addition a control group of 20 animals (10 animals per sex) should be added to the satellite study.
(5) Dose levels and dose selection. (i) In subchronic toxicity tests, it is desirable to determine a dose-response relationship as well as a NOEL. Therefore, at least three dose levels plus a control and, where appropriate, a vehicle control (corresponding to the concentration of vehicle at the highest dose level) group shall be used. Doses should be spaced appropriately to produce test groups with a range of toxic effects. The data should be sufficient to produce a dose-response curve.
(ii) The highest dose level should elicit signs of toxicity but not produce severe skin irritation or an incidence of fatality which would prevent a meaningful evaluation. If application of the test substance produces severe skin irritation, the concentration may be reduced, although this may result in a reduction in, or absence of, other toxic effects at the high dose level. If the skin has been badly damaged early in the study, it may be necessary to terminate the study and undertake a new one at lower concentrations.
(iii) The intermediate dose levels should be spaced to produce a gradation of toxic effects.
(iv) The lowest dose level should not produce any evidence of toxic effects.
(6) Preparation of animal skin. Shortly before testing, fur must be clipped from not less than 10% of the body surface area for application of the test substance. In order to dose approximately 10% of the body surface, the area starting at the scapulae (shoulders) to the wing of the ileum (hipbone) and half way down the flank on each side of the animal should be shaved. Shaving should be carried out approximately 24 hours before dosing. Repeated clipping or shaving is usually needed at approximately weekly intervals. When clipping or shaving the fur, care should be taken to avoid abrading the skin which could alter its permeability.
(7) Preparation of test substance. (i) Liquid test substances are generally used undiluted, except as indicated in paragraph (e)(5)(ii) of this section.
(ii) Solids should be pulverized when possible. The substance should be moistened sufficiently with water or, when necessary, a suitable vehicle to ensure good contact with the skin. When a vehicle is used, the influence of the vehicle on toxicity of, and penetration of the skin by, the test substance should be taken into account.
(iii) The volume of application should be kept constant, e.g., less than 300 mL for the rat; different concentrations of test solution shall be prepared for different dose levels.
(8) Administration of test substance. (i) The duration of exposure should be at least for 90 days.
(ii) Ideally, the animals should be treated with test substance for at least 6 hours per day on a 7-day per week basis. However, based on practical considerations, application on a 5-day per week basis is acceptable. Dosing should be conducted at approximately the same time each day. Start Printed Page 78788
(iii) The test substance must be applied uniformly over the treatment site.
(iv) The surface area covered may be less for highly toxic substances. As much of the area should be covered with as thin and uniform a film as possible.
(v) During the exposure period, the test substance must be held in contact with the skin with a porous gauze dressing (less than or equal to 8 ply). The test site must be further covered with nonirritating tape to retain the gauze dressing and the test substance and to ensure that the animals cannot ingest the test substance. Restrainers may be used to prevent the ingestion of the test substance, but complete immobilization is not recommended. The test substance may be wiped from the skin after the six-hour exposure period to prevent ingestion.
(9) Observation of animals. (i) Observations must be made at least twice each day for morbidity and mortality. Appropriate actions should be taken to minimize loss of animals to the study (e.g., necropsy or refrigeration of those animals found dead and isolation or sacrifice of weak or moribund animals). General clinical observations must be made at least once a day, preferably at the same time each day, taking into consideration the peak period of anticipated effects after dosing. The clinical condition of the animal should be recorded.
(ii) A careful clinical examination must be made at least once weekly. Observations should be detailed and carefully recorded, preferably using explicity defined scales. Observations should include, but not be limited to, evaluation of skin and fur, eyes and mucous membranes, respiratory and circulatory effects, autonomic effects such as salivation, central nervous system effects, including tremors and convulsions, changes in the level of activity, gait and posture, reactivity to handling or sensory stimuli, altered strength, and stereotypes or bizarre behavior (e.g., self-mutilation, walking backwards).
(iii) Signs of toxicity should be recorded as they are observed including the time of onset, degree and duration.
(iv) Individual weights of animals must be determined shortly before the test substance is administered, weekly thereafter, and at death.
(v) Food consumption must also be determined weekly if abnormal body weight changes are observed.
(vi) Moribund animals should be removed and sacrificed when noticed and the time of death should be recorded as precisely as possible.
(vii) At termination, all survivors in the control and treatment groups must be sacrificed.
(10) Clinical pathology. Hematology and clinical chemistry examinations must be made on all animals, including controls, of each sex in each group. The hematology and clinical chemistry parameters should be examined at terminal sacrifice at the end of the study. Overnight fasting of the animals prior to blood sampling is recommended. Overall, there is a need for a flexible approach in the measures examined, depending on the observed or expected effects from a chemical, and in the frequency of measures, depending on the duration of potential chemical exposures.
(i) Hematology. The recommended parameters are red blood cell count, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration, white blood cell count, differential leukocyte count, platelet count, and a measure of clotting potential, such as prothrombin time or activated partial thromboplastin time.
(ii) Clinical chemistry. (A) Parameters which are considered appropriate to all studies are electrolyte balance, carbohydrate metabolism, and liver and kidney function. The selection of specific tests will be influenced by observations on the mode of action of the substance and signs of clinical toxicity.
(B) The recommended clinical chemistry determinations are potassium, sodium, glucose, total cholesterol, urea nitrogen, creatinine, total protein and albumin. More than 2 hepatic enzymes, (such as alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, sorbitol dehydrogenase, or gamma glutamyl transpeptidase) should also be measured. Measurements of additional enzymes (of hepatic or other origin) and bile acids, may also be useful.
(C) If a test chemical has an effect on the hematopoietic system, reticulocyte counts and bone marrow cytology may be indicated.
(D) Other determinations that should be carried out if the test chemical is known or suspected of affecting related measures include calcium, phosphorus, fasting triglycerides, hormones, methemoglobin, and cholinesterases.
(iii) Optionally, the following urinalysis determinations could be performed during the last week of the study using timed urine volume collection: appearance, volume, osmolality or specific gravity, pH, protein, glucose and blood/blood cells.
(11) Ophthalmological examination. Using an ophthalmoscope or an equivalent device, ophthalmological examinations must be made on all animals prior to the administration of the test substance and on all high dose and control groups at termination. If changes in the eyes are detected, all animals in the other dose groups must be examined.
(12) Gross necropsy. (i) All animals must be subjected to a full gross necropsy which includes examination of the external surface of the body, all orifices, and the cranial, thoracic and abdominal cavities and their contents.
(ii) The liver, brain, kidneys, spleen, adrenals, testes, epididymides, uterus, ovaries, thymus and heart must be trimmed and weighed wet, as soon as possible after dissection.
(iii) The following organs and tissues, or representative samples thereof, must be preserved in a suitable medium for possible future histopathological examination:
(A) Digestive system—salivary glands, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, liver, pancreas, gallbladder (when present).
(B) Nervous system—brain (multiple sections, including cerebrum, cerebellum and medulla/pons), pituitary, peripheral nerve (sciatic or tibial, preferably in close proximity to the muscle), spinal cord (three levels, cervical, mid-thoracic and lumbar), eyes (retina, optic nerve).
(C) Glandular system—adrenals, parathyroid, thyroid.
(D) Respiratory system—trachea, lungs, pharynx, larynx, nose.
(E) Cardiovascular/Hematopoietic system—aorta, heart, bone marrow (and/or fresh aspirate), lymph nodes (preferably one lymph node covering the route of administration and another one distant from the route of administration to cover systemic effects), spleen, thymus.
(F) Urogenital system—kidneys, urinary bladder, prostate, testes, epididymides, seminal vesicle(s), uterus, ovaries, female mammary gland.
(G) Other—all gross lesions and masses, skin (both treated and adjacent untreated areas).
(13) Histopathology. (i) The following histopathology must be performed:
(A) Full histopathology on the organs and tissues, listed in paragraph (e)(12)(iii) of this section, of all animals in the control and high dose groups and all animals that died or were sacrificed during the study.
(B) All gross lesions in all animals.
(C) Target organs in all animals. Start Printed Page 78789
(D) When a satellite group is used, histopathology must be performed on tissues and organs identified as showing toxic effects in the treated groups.
(ii) If excessive early deaths or other problems occur in the high dose group compromising the significance of the data, the next dose level must be examined for complete histopathology.
(iii) An attempt should be made to correlate gross observations with microscopic findings.
(iv) Tissues and organs designated for microscopic examination should be fixed in 10% buffered formalin or a recognized suitable fixative as soon as necropsy is performed and no less than 48 hours prior to trimming.
(f) Data and reporting—(1) Treatment of results. (i) Data must be summarized in tabular form, showing for each test group, number of animals at the start of the test, the number of animals showing lesions, the types of lesions and the percentage of animals displaying each type of lesion.
(ii) When applicable, all observed results, qualitative and quantitative, should be evaluated by an appropriate and generally acceptable statistical method. Any generally accepted statistical method should be used; the statistical methods including significance criteria should be selected during the design of the study.
(2) Evaluation of study results. The findings of a subchronic dermal toxicity study should be evaluated in conjunction with the findings of preceding studies and considered in terms of toxic effects and the necropsy and histopathological findings. The evaluation should include the relationship between the dose of the test substance, the incidence and severity of abnormalities including behavioral and clinical abnormalities, gross lesions, identified target organs, body weight changes, effect on mortality, and any other general or specific toxic effects. A properly conducted 90-day subchronic dermal study should provide information on the effects of repeated application of a substance and a satisfactory estimation of a NOEL. It also can indicate the need for an additional longer-term study and provide information on the selection of dose levels.
(3) Test report. In addition to reporting requirements specified under EPA Good Laboratory Practice Standards at 40 CFR part 792, subpart J, the following specific information must be reported:
(i) Test substance characterization should include:
(A) Chemical identification.
(B) Lot or batch numbers.
(C) Physical properties.
(D) Purity/impurities.
(ii) Identification and composition of any vehicle if used.
(iii) Test system should contain data on:
(A) Species and strain of animals used and rationale for selection if other than that recommended.
(B) Age including body weight data and sex.
(C) Test environment including cage conditions, ambient temperature, humidity, and light/dark periods.
(D) Identification of animal diet.
(E) Acclimation period.
(iv) Test procedure should include the following data:
(A) Method of randomization used.
(B) Full description of experimental design and procedure.
(C) Dose regime including levels, method, and volume.
(v) Test results should include:
(A) Group animal data. Tabulation of toxic response data by species, strain, sex and exposure level for:
(1) Number of animals exposed.
(2) Number of animals showing signs of toxicity.
(3) Number of animals dying.
(B) Individual animal data. Data should be presented as summary (group mean) as well as for individual animals.
(1) Date of death during the study or whether animals survived to termination.
(2) Date of observation of each abnormal sign and its subsequent course.
(3) Body weight data.
(4) Feed consumption data, when collected.
(5) Results of ophthalmological examination.
(6) Results of hematological tests performed.
(7) Results of clinical chemistry tests performed.
(8) Results of urinalysis, when performed.
(9) Results of observations made.
(10) Necropsy findings, including absolute and relative (to body weight) organ weight data.
(11) Detailed description of all histopathological findings.
(12) Statistical treatment of results, where appropriate.
(g) Quality control. A system must be developed and maintained to assure and document adequate performance of laboratory equipment. The study must be conducted in compliance with the Good Laboratory Practice (GLP) regulations.
(h) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., NW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Organization for Economic Cooperation and Development. Guidelines for Testing of Chemicals, Section 4-Health Effects, Part 411 Subchronic Toxicity Studies, Paris, 1981.
(2) Weingand K, Brown G, Hall R et al. (1996). Harmonization of Animal Clinical Pathology Testing in Toxicity and Safety Studies. Fundam. & Appl. Toxicol. 29:198-201.
TSCA reproduction/developmental toxicity screening test.(a) Scope—(1) Applicability. This section is intended to meet testing requirements of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides, and Toxic Substances (OPPTS) harmonized test guideline 870.3550 (July 2000, final guidelines). This source is available at the address in paragraph (h) of this section.
(b) Purpose. (1) This guideline is designed to generate limited information concerning the effects of a test substance on male and female reproductive performance such as gonadal function, mating behavior, conception, development of the conceptus, and parturition. It is not an alternative to, nor does it replace, the existing comprehensive test standards in §§ 799.9370 and 799.9380.
(2) This screening test guideline can be used to provide initial information on possible effects on reproduction and/or development, either at an early stage of assessing the toxicological properties of chemicals, or on chemicals of high concern. It can also be used as part of a set of initial screening tests for existing chemicals for which little or no toxicological information is available, as a dose range finding study for more extensive reproduction/developmental studies, or when otherwise considered relevant.
(3) This test does not provide complete information on all aspects of reproduction and development. In particular, it offers only limited means of detecting postnatal manifestations of prenatal exposure, or effects that may be induced during postnatal exposure. Due (amongst other reasons) to the relatively small numbers of animals in the dose groups, the selectivity of the end points, Start Printed Page 78790and the short duration of the study, this method will not provide evidence for definite claims of no effects.
(c) Definitions. The definitions in section 3 of TSCA and in 40 CFR Part 792—Good Laboratory Practice Standards apply to this section. The following definitions also apply to this section.
Dosage is a general term comprising of dose, its frequency and the duration of dosing.
Dose is the amount of test substance administered. Dose is expressed as weight (g, mg) as weight of test substance per unit weight of test animal (e.g., mg/kg), or as constant dietary concentration parts per million (ppm).
No-observed-effects level (NOEL) is the maximum dose used in a study which produces no adverse effects. The NOEL is expressed in terms of the weight of a test substance given daily per unit weight of test animal (milligrams per kilograms per day).
(d) Principle of the test. (1) The test substance is administered in graduated doses to several groups of males and females. Males should be dosed for a minimum of four weeks and up to and including the day before scheduled sacrifice (this includes a minimum of two weeks prior to mating, during the mating period and, approximately, two weeks post-mating). In view of the limited pre-mating dosing period in males, fertility may not be a particular sensitive indicator of testicular toxicity. Therefore, a detailed histological examination of the testes is essential. The combination of a pre-mating dosing period of two weeks and subsequent mating/fertility observations with an overall dosing period of at least four weeks, followed by detailed histopathology of the male gonads, is considered sufficient to enable detection of the majority of effects on male fertility and spermatogenesis.
(2) Females should be dosed throughout the study. This includes two weeks prior to mating (with the objective of covering at least two complete oestrous cycles), the variable time to conception, the duration of pregnancy and at least four days after delivery, up to and including the day before scheduled sacrifice.
(3) Duration of study, following acclimatization, is dependent on the female performance and is approximately 54 days, (at least 14 days premating, (up to) 14 days mating, 22 days gestation, 4 days lactation).
(4) During the period of administration, the animals are observed closely each day for signs of toxicity. Animals which die or are sacrificed during the test period are necropsied and, at the conclusion of the test, surviving animals are sacrificed and necropsied.
(e) Description of the method—(1) Selection of animal species. This test standard is designed for use with the rat. If other species are used, appropriate modifications will be necessary. Strains with low fecundity or well-known high incidence of developmental defects should not be used. Healthy virgin animals, not subjected to previous experimental procedures, should be used. The test animals should be characterized as to species, strain, sex, weight and/or age. At the commencement of the study the weight variation of animals used should be minimal and not exceed 20% of the mean weight of each sex.
(2) Housing and feeding conditions. (i) The temperature in the experimental animal room should be 22 °C (± 3°). Although the relative humidity should be at least 30% and preferably not exceed 70% other than during room cleaning, the aim should be 50-60%. Lighting should be artificial, the sequence being 12 hours light, 12 hours dark. For feeding, conventional laboratory diets may be used with an unlimited supply of drinking water. The choice of diet may be influenced by the need to ensure a suitable admixture of a test substance when administered by this method.
(ii) Animals may be housed individually or be caged in small groups of the same sex; for group caging, no more than five animals should be housed per cage. Mating procedures should be carried out in cages suitable for the purpose. Pregnant females should be caged individually and provided with nesting materials.
(3) Preparation of the animals. Healthy young adult animals must be randomly assigned to the control and treatment groups. Cages should be arranged in such a way that possible effects due to cage placement are minimized. The animals must be uniquely identified and kept in their cages for at least five days prior to the start of the study to allow for acclimatization to the laboratory conditions.
(4) Preparation of doses. (i) It is recommended that the test substance be administered orally unless other routes of administration are considered more appropriate. When the oral route is selected, the test compound is usually administered by gavage; however, alternatively, test compounds may be administered via the diet or drinking water.
(ii) Where necessary, the test substance is dissolved or suspended in a suitable vehicle. It is recommended that, wherever possible, the use of an aqueous solution/suspension be considered first, followed by consideration of a solution/emulsion in oil (e.g., corn oil) and then by possible solution in other vehicles. For vehicles other than water the toxic characteristics of the vehicle must be known. The stability of the test substance in the vehicle should be determined.
(f) Procedure—(1) Number and sex of animals. It is recommended that each group be started with at least 10 animals of each sex. Except in the case of marked toxic effects, it is expected that this will provide at least 8 pregnant females per group which normally is the minimum acceptable number of pregnant females per group. The objective is to produce enough pregnancies and offspring to assure a meaningful evaluation of the potential of the substance to affect fertility, pregnancy, maternal and suckling behaviour, and growth and development of the F1 offspring from conception to day 4 post-partum.
(2) Dosage. (i) Generally, at least three test groups and a control group should be used. Dose levels may be based on information from acute toxicity tests or on results from repeated dose studies. Except for treatment with the test substance, animals in the control group should be handled in an identical manner to the test group subjects. If a vehicle is used in administering the test substance, the control group should receive the vehicle in the highest volume used.
(ii) Dose levels should be selected taking into account any existing toxicity and (toxico-) kinetic data available for the test compound or related materials. The highest dose level should be chosen with the aim of inducing toxic effects but not death or severe suffering. Thereafter, a descending sequence of dose levels should be selected in order to demonstrate any dose response relationships and no adverse effects at the lowest dose level. Two to four fold intervals are frequently optimal for setting the descending dose levels and addition of a fourth test group is often preferable to using very large intervals (e.g., more than a factor of 10) between dosages.
(3) Limit test. If an oral study at one dose level of at least 1000 mg/kg body weight/day or, for dietary or drinking water administration, an equivalent percentage in the diet, or drinking water using the procedures described for this study, produces no observable toxic effects and if toxicity would not be expected based upon data from Start Printed Page 78791structurally related compounds, then a full study using several dose levels may not be considered necessary. The limit test applies except when human exposure indicates the need for a higher oral dose level to be used. For other types of administration, such as inhalation or dermal application, the physical chemical properties of the test substance often may dictate the maximum attainable concentration.
(4) Administration of doses. (i) The animals must be dosed with the test substance daily for seven days a week. When the test substance is administered by gavage, this should be done in a single dose to the animals using a stomach tube or a suitable intubation cannula. The maximum volume of liquid that can be administered at one time depends on the size of the test animal. The volume should not exceed 1 ml/100 g body weight, except in the case of aqueous solutions where 2 ml/100 g body weight may be used. Except for irritating substances which will normally reveal exacerbated effects with higher concentrations, variability in test volume should be minimized by adjusting the concentration to ensure a constant volume at all dose levels.
(ii) For substances administered via the diet or drinking water, it is important to ensure that the quantities of the test substance involved do not interfere with normal nutrition or water balance. When the test substance is administered in the diet either a constant dietary concentration (parts per million (ppm)) or a constant dose level in terms of the animals' body weight may be used; the alternative used must be specified. For a substance administered by gavage, the dose should be given at similar times each day, and adjusted at least weekly to maintain a constant dose level in terms of animal body weight.
(5) Experimental schedule. (i) Dosing of both sexes should begin at least 2 weeks prior to mating, after they have been acclimatized for at least five days. The study should be scheduled in such a way that mating begins soon after the animals have attained full sexual maturity. This may vary slightly for different strains of rats in different laboratories, e.g., Sprague Dawley rats 10 weeks of age, Wistar rats about 12 weeks of age. Dams with offspring should be sacrificed on day 4 post-partum, or shortly thereafter. The day of birth (viz. when parturition is complete) is defined as day 0 post-partum. Females showing no-evidence of copulation are sacrificed 24-26 days after the last day of the mating period. Dosing is continued in both sexes during the mating period. Males should further be dosed after the mating period at least until the minimum total dosing period of 28 days has been completed. They are then sacrificed, or, alternatively, are retained and continued to be dosed for the possible conduction of a second mating if considered appropriate.
(ii) Daily dosing of the parental females should continue throughout pregnancy and at least up to, and including, day 3 post-partum or the day before sacrifice. For studies where the test substance is administered by inhalation or by the dermal route, dosing should be continued at least up to, and including, day 19 of gestation.
(iii) The experimental schedule is given in the following figure 1.
(6) Mating procedure. Normally, 1:1 (one male to one female) matings should be used in this study. Exceptions can arise in the case of occasional deaths of males. The female should be placed with the same male until pregnancy occurs or two weeks have elapsed. Each morning the females should be examined for the presence of sperm or a vaginal plug. Day 0 of pregnancy is defined as the day a vaginal plug or sperm is found.
(7) Observations. (i) Throughout the test period, general clinical observations should be made at least once a day, and more frequently when signs of toxicity are observed. They should be made preferably at the same time(s) each day, considering the peak period of anticipated effects after dosing. Pertinent behavioural changes, signs of difficult or prolonged parturition and all signs of toxicity, including mortality, should be recorded. These records should include time of onset, degree and duration of toxicity signs. Start Printed Page 78792
(ii) The duration of gestation should be recorded and is calculated from day 0 of pregnancy. Each litter should be examined as soon as possible after delivery to establish the number and sex of pups, stillbirths, live births, runts (pups that are significantly smaller than corresponding control pups) and the presence of gross abnormalities.
(iii) Live pups should be counted and sexed and litters weighed within 24 hours of parturition (day 1) and on day 4 post-partum. In addition to the observations on parent animals, described by paragraph (f)(7) of this section, any abnormal behaviour of the offspring should be recorded.
(8) Body weight and food/water consumption. (i) Males and females should be individually weighed on the first day of dosing, at least weekly thereafter, and at termination. During pregnancy, females should be weighed on days 0, 7, 14 and 20 and within 24 hours of parturition (day 1) and day 4 post-partum.
(ii) During pre-mating, pregnancy and lactation, food consumption should be measured at least weekly. The measurement of food consumption during mating is optional. Water consumption during these periods should also be measured when the test substance is administered via drinking water.
(9) Pathology—(i) Gross necropsy. (A) At the time of sacrifice or death during the study, the adult animals should be examined macroscopically for any abnormalities or pathological changes. Special attention should be paid to the organs of the reproductive system. The number of implantation sites should be recorded. Corpora lutea should be counted.
(B) The testes and epididymides of all male adult animals should be weighed.
(C) Dead pups and pups sacrificed at day 4 post-partum, or shortly thereafter, should, at least, be carefully examined externally for gross abnormalities.
(D) The ovaries, testes, epididymides, accessory sex organs and all organs showing macroscopic lesions of all adult animals should be preserved. Formalin fixation is not recommended for routine examination of testes and epididymides. An acceptable method is the use of Bouin's fixative for these tissues.
(ii) Histopathology. (A) Detailed histological examination should be performed on the ovaries, testes and epididymides of the animals of the highest dose group and the control group. The other preserved organs may be examined when necessary. Examinations should be extended to the animals of other dosage groups when changes are seen in the highest dose group.
(B) Detailed testicular histopathological examination (e.g., using Bouin's fixative, paraffin embedding and transverse sections of 4-5 ±m thickness) should be conducted with special emphasis on stages of spermatogenesis and histopathology interstitial testicular cell structure. The evaluation should identify treatment-related effects such as retained spermatids, missing germ cell layers or types, multinucleated giant cells or sloughing of spermatogenic cells into the lumen (the specifications for the evaluation are discussed in paragraph (g)(2) of this section). Examination of the intact epididymis should include the caput, corpus, and cauda, which can be accomplished by evaluation of a longitudinal section. The epididymis should be evaluated for leukocyte infiltration, change in prevalence of cell types, aberrant cell types, and phagocytosis of sperm. PAS and hematoxylin staining may be used for examination of the male reproductive organs. Histopathological examination of the ovary should detect qualitative depletion of the primordial follicle population.
(g) Data and reporting—(1) Data. Individual animal data should be provided. Additionally, all data should be summarised in tabular form, showing for each test group the number of animals at the start of the test, the number of animals found dead during the test or sacrificed for humane reasons, the time of any death or humane sacrifice, the number of fertile animals, the number of pregnant females, the number of animals showing signs of toxicity, a description of the signs of toxicity observed, including time of onset, duration, and severity of any toxic effects, the types of histopathological changes, and all relevant litter data.
(2) Evaluation of results. (i) The findings of this toxicity study should be evaluated in terms of the observed effects, necropsy and microscopic findings. This evaluation must include the relationship between the dose of the test substance and the presence or absence, incidence and severity of abnormalities, including gross lesions, identified target organs, infertility, clinical abnormalities, affected reproductive and litter performance, body weight changes, effects on mortality and any other toxic effects.
(ii) Because of the short period of treatment of the male, the histopathology of the testis and epididymus must be considered along with the fertility data, when assessing male reproductive effects.
(iii) Due to the limited dimensions of the study, statistical analysis in the form of tests for “significance” are of limited value for many endpoints, especially reproductive endpoints. If statistical analyses are used then the method chosen should be appropriate for the distribution of the variable examined, and be selected prior to the start of the study. Because of the small group size, the use of historic control data (e.g., for litter size), where available, may also be useful as an aid to the interpretation of the study.
(3) Test report. The test report must include the following information:
(i) Test substance:
(A) Physical nature and, where relevant, physicochemical properties.
(B) Identification data.
(ii) Vehicle (if appropriate): Justification for choice of vehicle if other than water.
(iii) Test animals:
(A) Species/strain used.
(B) Number, age and sex of animals.
(C) Source, housing conditions, diet, etc.
(D) Individual weights of animals at the start of the test.
(iv) Test conditions:
(A) Rationale for dose level selection.
(B) Details of test substance formulation/diet preparation, achieved concentrations, stability and homogeneity of the preparation.
(C) Details of the administration of the test substance.
(D) Conversion from diet/drinking water test substance concentration (parts per million (ppm)) to the actual dose (mg/kg body weight/day), if applicable.
(E) Details of food and water quality.
(v) Results (toxic response data by sex and dose):
(A) Time of death during the study or whether animals survived to termination.
(B) Nature, severity and duration of clinical observations (whether reversible or not).
(C) Body weight/body weight change data.
(D) Food consumption and water consumption, if applicable.
(E) Effects on reproduction, including information on mating/precoital interval, fertility, fecundity and gestation duration.
(F) Effects on offspring, including number of pups born (live and dead), sex ratio, postnatal growth (pup weights) and survival (litter size), gross abnormalities and clinical observations during lactation. Start Printed Page 78793
(G) Body weight at termination and organ weight data for the parental animals.
(H) Necropsy data, including number of implantations and number of corpora lutea.
(I) Calculations of pre- and postimplantation loss.
(J) Detailed description of histopathological findings.
(K) Statistical treatment of results, where appropriate.
(vi) Discussion of results.
(vii) Conclusions.
(4) Interpretation of results. The study will provide evaluations of reproduction/developmental toxicity associated with administration of repeated doses. It could provide an indication of the need to conduct further investigations and provides guidance in the design of subsequent studies.
(h) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., SW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) OECD (1995). Reproduction/Developmental Toxicity Screening Test, OECD 421, OECD Guidelines for Testing of Chemicals.
(2) [Reserved]
TSCA combined repeated dose toxicity study with the reproduction/developmental toxicity screening test.(a) Scope—(1) Applicability. This section is intended to meet testing requirements of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides and Toxic Substances (OPPTS) harmonized test guideline 870.3650 (July 2000, final guidelines). This source is available at the address in paragraph (h) of this section.
(b) Purpose. (1) This screening test provides limited information on systemic toxicity, neurotoxicity, and/or immunotoxicity following repeated exposure over a limited time period. In addition, it can be used to provide initial information on possible effects on male and female reproductive performance such as gonadal function, mating behavior, conception, development of the conceptus, and parturition. It is not an alternative to, nor does it replace, the existing test guidelines in §§ 799.9370, 799.9380, 799.9620, and 799.9780 of this part.
(2) This test does not provide complete information on all aspects of reproduction and development. In particular, it offers only limited means of detecting postnatal manifestations of prenatal exposure, or effects that may be induced during postnatal exposure. Due (amongst other reasons) to the selectivity of the end points, and the short duration of the study, this method will not provide evidence for definite claims of no reproduction/developmental effects.
(3) This test can be used to provide initial information either at an early stage of assessing the toxicological properties of chemicals, or chemicals of high concern. It can also be used as part of a set of initial screening tests for existing chemicals for which little or no toxicological information is available or when otherwise considered relevant. It also can serve as an alternative to conducting two separate screening tests for repeated dose toxicity as described in § 799.9305 of this part and reproductive/developmental toxicity as described in § 799.9355 of this part.
(c) Definitions. The definitions in section 3 of TSCA and in 40 CFR Part 792—Good Laboratory Practice Standards apply to this section. The following definitions also apply to this section.
Dosage is a general term comprising dose, its frequency and the duration of dosing.
Dose is the amount of test substance administered. Dose is expressed as weight (g, gm) or as weight of test substance per unit weight of test animal (e.g., mg/kg), or as constant dietary concentration (parts per million (ppm)).
No-observed-effects level (NOEL) is the maximum dose used in a study which produces no adverse effects. The NOEL is expressed in terms of the weight of a test substance given daily per unit weight of test animal (milligrams per kilogram per day).
(d) Principle of the test. (1) The test substance must be administered in graduated doses to several groups of males and females. Males should be dosed for a minimum of 4 weeks, up to and including the day before scheduled sacrifice (this includes a minimum of 2 weeks prior to mating, during the mating period and, approximately, 2 weeks post mating). In view of the limited pre-mating dosing period in males, fertility may not be a particularly sensitive indicator of testicular toxicity. Therefore, a detailed histological examination of the testes is essential. The combination of a pre-mating dosing period of 2 weeks and subsequent mating/fertility observations with an overall dosing period of at least 4 weeks, followed by detailed histopathology of the male gonads, is considered sufficient to enable detection of the majority of effects on male fertility and spermatogenesis.
(2) Females should be dosed throughout the study. This includes 2 weeks prior to mating (with the objective of covering at least two complete oestrous cycles), the variable time to conception, the duration of pregnancy and at least 4 days after delivery, up to and including the day before scheduled sacrifice.
(3) Duration of study, following acclimatization, is dependent on the female performance and is approximately 54 days, (at least 14 days pre-mating, (up to) 14 days mating, 22 days gestation, 4 days lactation).
(4) During the period of administration, the animals are observed closely each day for signs of toxicity. Animals which die or are sacrificed during the test are necropsied and, at the conclusion of the test, surviving animals are sacrificed and necropsied.
(e) Description of the method—(1) Selection of animal species. This test guideline is designed for use with the rat. If other species are used, appropriate modifications will be necessary. Strains with low fecundity or well-known high incidence of developmental defects should not be used. Healthy virgin animals, not subjected to previous experimental procedures, should be used. The test animals should be characterised as to species, strain, sex, weight and/or age. At the commencement of the study the weight variation of animals used should be minimal and not exceed ± 20% of the mean weight of each sex. Where the study is conducted as a preliminary study to a long-term or a full-generation study, preferably animals from the same strain and source should be used in both studies.
(2) Housing and feeding conditions. (i) The temperature in the experimental animal room should be 22 °C (± 3°). The relative humidity should be at least 30% and preferably not exceed 70% other than during room cleaning. Lighting should be artificial, the sequence being 12 hours light, 12 hours dark. For feeding, conventional laboratory diets may be used with an unlimited supply of drinking water. The choice of diet may be influenced by the need to ensure a suitable admixture of a test substance when administered by this method.
(ii) Animals may be housed individually or be caged in small groups of the same sex; for group caging, no more than five animals should be housed per cage. Mating procedures Start Printed Page 78794should be carried out in cages suitable for the purpose. Pregnant females should be caged individually and provided with nesting materials.
(3) Preparation of the animals. Healthy young adult animals must be randomised and assigned to the treatment groups and cages. Cages should be arranged in such a way that possible effects due to cage placements are minimized. The animals must be uniquely identified and kept in their cages for at least 5 days prior to the start of the study to allow for acclimatisation to the laboratory conditions.
(4) Preparation of doses. (i) It is recommended that the test substance be administered orally unless other routes of administration are considered more appropriate. When the oral route is selected, the test compound is usually administered by gavage; however, alternatively, test compounds may also be administered via the diet or drinking water.
(ii) Where necessary, the test substance is dissolved or suspended in a suitable vehicle. It is recommended that, wherever possible, the use of an aqueous solution/suspension be considered first, followed by consideration of a solution/emulsion in oil (e.g., corn oil) and then by possible solution in other vehicles. For non-aqueous vehicles the toxic characteristics of the vehicle must be known. The stability of the test substance in the vehicle should be determined.
(f) Procedure—(1) Number and sex of animals. It is recommended that each group be started with at least 10 animals of each sex. Except in the case of marked toxic effects, it is expected that this will provide at least eight pregnant females per group which normally is the minimum acceptable number of pregnant females per group. The objective is to produce enough pregnancies and offspring to assure a meaningful evaluation of the potential of the substance to affect fertility, pregnancy, maternal and suckling behaviour, and growth and development of the F1 offspring from conception to day 4 post-partum. If interim sacrifices are planned, the number should be increased by the number of animals scheduled to be sacrificed before the completion of the study. Consideration should be given to an additional satellite group of five animals per sex in the control and the top dose group for observation of reversibility, persistence or delayed occurrence of systemic toxic effects, for at least 14 days post treatment. Animals of the satellite groups must not be mated and, consequently, must not used for the assessment of reproduction/developmental toxicity.
(2) Dosage. (i) Generally, at least three test groups and a control group should be used. If there are no suitable general toxicity data available, a range finding study may be performed to aid the determination of the doses to be used. Except for treatment with the test substance, animals in the control group should be handled in an identical manner to the test group subjects. If a vehicle is used in administering the test substance, the control group should receive the vehicle in the highest volume used.
(ii) Dose levels should be selected taking into account any existing toxicity and (toxico-) kinetic data available for the test compound or related materials. It should also be taken into account that there may be differences in sensitivity between pregnant and non-pregnant animals. The highest dose level should be chosen with the aim of inducing toxic effects but not death nor obvious suffering. Thereafter, a descending sequence of dose levels should be selected with a view to demonstrating any dosage related response and no adverse effects at the lowest dose level. Two- to four-fold intervals are frequently optimum and addition of a fourth test group is often preferable to using very large intervals (e.g., more than a factor of 10) between dosages.
(3) Limit test. If an oral study at 1-dose level of at least 1000 mg/kg body weight/day or, for dietary administration, an equivalent percentage in the diet, or drinking water (based upon body weight determinations), using the procedures described for this study, produces no observable toxic effects and if toxicity would not be expected based upon data from structurally related compounds, then a full study using several dose levels may not be considered necessary. The limit test applies except when human exposure indicates the need for a higher dose level to be used. For other types of administration, such as inhalation or dermal application, the physical chemical properties of the test substance often may dictate the maximum attainable exposure.
(4) Administration of doses. (i) The animals are dosed with the test substance daily for 7 days a week. When the test substance is administered by gavage, this should be done in a single dose to the animals using a stomach tube or a suitable intubation cannula. The maximum volume of liquid that can be administered at one time depends on the size of the test animal. The volume should not exceed 1 ml/100 g body weight, except in the case of aqueous solutions where 2 ml/100 g body weight may be used. Except for irritating or corrosive substances which will normally reveal exacerbated effects with higher concentrations, variability in test volume should be minimized by adjusting the concentration to ensure a constant volume at all dose levels.
(ii) For substances administered via the diet or drinking water, it is important to ensure that the quantities of the test substance involved do not interfere with normal nutrition or water balance. When the test substance is administered in the diet either a constant dietary concentration (parts per million (ppm)) or a constant dose level in terms of the animals' body weight may be used; the alternative used must be specified. For a substance administered by gavage, the dose should be given at similar times each day, and adjusted at least weekly to maintain a constant dose level in terms of animal body weight.
(5) Experimental schedule. (i) Dosing of both sexes should begin 2 weeks prior to mating, after they have been acclimatized for at least 5 days. The study should be scheduled in such a way that mating begins soon after the animals have attained full sexual maturity. This may vary slightly for different strains of rats in different laboratories, e.g., Sprague Dawley rats 10 weeks of age, Wistar rats about 12 weeks of age. Dams with offspring should be sacrificed on day 4 post-partum, or shortly thereafter. In order to allow for overnight fasting of dams prior to blood collection (if this option is preferred), dams and their offspring need not necessarily be sacrificed on the same day. The day of birth (viz. when parturition is complete) is defined as day 0 post-partum. Females showing no-evidence of copulation are sacrificed 24-26 days after the last day of the mating period. Dosing is continued in both sexes during the mating period. Males should further be dosed after the mating period at least until the minimum total dosing period of 28 days has been completed. They are then sacrificed, or, alternatively, are retained and continued to be dosed for the possible conduction of a second mating if considered appropriate.
(ii) Daily dosing of the parental females should continue throughout pregnancy and at least up to, and including, day 3 post-partum or the day before sacrifice. For studies where the test substance is administered by inhalation or by the dermal route, dosing should be continued at least up to, and including, day 19 of gestation.
(iii) Animals in a satellite group scheduled for follow-up observations, if Start Printed Page 78795included, must not mated. They should be kept at least for a further 14 days after the first scheduled sacrifice of dams, without treatment to detect delayed occurrence, or persistence of, or recovery from toxic effects.
(iv) The experimental schedule is given in the following figure 1.
(6) Mating procedure. Normally, 1:1 (one male to one female) matings should be used in this study. Exceptions can arise in the case of occasional deaths of males. The female should be placed with the same male until pregnancy occurs or 2 weeks have elapsed. Each morning the females should be examined for the presence of sperm or a vaginal plug. Day 0 of pregnancy is defined as the day a vaginal plug or sperm is found. In case pairing was unsuccessful, re-mating of females with proven males of the same group could be considered.
(7) Observations. (i) General clinical observations should be made at least once a day, preferably at the same time(s) each day and considering the peak period of anticipated effects after dosing. The health condition of the animals should be recorded. At least twice daily all animals must be observed for morbidity and mortality.
(ii) Once before the first exposure (to allow for within-subject comparisons), and at least once a week thereafter, detailed clinical observations should be made in all animals. These observations should be made outside the home cage in a standard arena and preferably at the same time, each day. They should be carefully recorded; preferably using scoring systems, explicitly defined by the testing laboratory. Effort should be made to ensure that variations in the test conditions are minimal and that observations are preferably conducted by observers unaware of the treatment. Signs noted should include, but not be limited to, changes in skin, fur, eyes, mucous membranes, occurrence of secretions and excretions and autonomic activity (e.g., lacrimation, piloerection, pupil size, unusual respiratory pattern). Changes in gait, posture and response to handling as well as the presence of clonic or tonic movements, stereotypies (e.g., excessive grooming, repetitive circling), difficult or prolonged parturition or bizarre behaviour (e.g., self-mutilation, walking backwards) should also be recorded.
(iii) At one time during the study, sensory reactivity to stimuli of different modalities (e.g., auditory, visual and proprioceptive stimuli) assessment of grip strength and motor activity assessment should be conducted in five males and five females, randomly selected from each group. Further details of the procedures that could be followed are given in the respective references. However, alternative procedures than those referenced could also be used. In males, these functional observations should be made towards the end of their dosing period, shortly before scheduled sacrifice but before blood sampling for hematology or clinical chemistry. Females should be in a physiologically similar state during these functional tests and should preferably be tested during lactation, shortly before scheduled sacrifice. In order to avoid hypothermia of pups, dams should be removed from the pups for not more than 30 to 40 minutes. Examples of procedures for observation are described in the references in paragraphs (h)(3), (h)(4), (h)(5), (h)(6), and (h)(7) of this section.
(iv) Functional observations made once towards the end of the study may be omitted when the study is conducted as a preliminary study to a subsequent subchronic (90-day) or long-term study. In that case, the functional observations should be included in this follow-up study. On the other hand, the availability of data on functional observations from this repeated dose study may enhance the ability to select dose levels for a subsequent subchronic or long-term study.
(v) Functional observations may also be omitted for groups that otherwise reveal signs of toxicity to an extent that would significantly interfere with the functional test performance.
(vi) The duration of gestation should be recorded and is calculated from day 0 of pregnancy. Each litter should be examined as soon as possible after delivery to establish the number and sex of pups, stillbirths, live births, runts (pups that are significantly smaller than corresponding control pups), and the presence of gross abnormalities.
(vii) Live pups should be counted and sexed and litters weighed within 24 hours of parturition (day 0 or 1 post-partum) and on day 4 post-partum. In addition to the observations on parental animals, described by paragraphs (f)(7)(ii) and (f)(7)(iii) of this section, any abnormal behaviour of the offspring should be recorded.
(8) Body weight and food/water consumption. (i) Males and females should be weighed on the first day of dosing, at least weekly thereafter, and at termination. During pregnancy, females should be weighed on days 0, 7, 14 and 20 and within 24 hours of parturition (day 0 or 1 post-partum), and day 4 post-partum. These observations should be reported individually for each adult animal. Start Printed Page 78796
(ii) During pre-mating, pregnancy and lactation, food consumption should be measured at least weekly. The measurement of food consumption during mating is optional. Water consumption during these periods should also be measured, when the test substance is administered by that medium.
(9) Hematology. (i) Once during the study, the following hematological examinations should be made in five males and five females randomly selected from each group: hematocrit, hemoglobin concentration, erythrocyte count, total and differential leucocyte count, platelet count and a measure of blood clotting time/potential.
(ii) Blood samples should be taken from a named site. Females should be in a physiologically similar state during sampling. In order to avoid practical difficulties related to the variability in the onset of gestation, blood collection in females may be done at the end of the pre-mating period as an alternative to sampling just prior to, or as part of, the procedure for sacrificing the animals. Blood samples of males should preferably be taken just prior to, or as part of, the procedure for sacrificing the animals. Alternatively, blood collection in males may also be done at the end of the pre-mating period when this time point was preferred for females.
(iii) Blood samples should be stored under appropriate conditions.
(10) Clinical biochemistry. (i) Clinical biochemistry determinations to investigate major toxic effects in tissues and, specifically, effects on kidney and liver, should be performed on blood samples obtained from the selected five males and five females of each group. Overnight fasting of the animals prior to blood sampling is recommended[1] . Investigations of plasma or serum must include sodium, potassium, glucose, total cholesterol, urea, creatinine, total protein and albumin, at least two enzymes indicative of hepatocellular effects (such as alanine aminotransferase, aspartate aminotransferase and sorbitol dehydrogenase) and bile acids. Measurements of additional enzymes (of hepatic or other origin) may provide useful information under certain circumstances.
(ii) Optionally, the following urinalysis determinations could be performed in five randomly selected males of each group during the last week of the study using timed urine volume collection; appearance, volume, osmolality or specific gravity, pH, protein, glucose and blood or blood cells.
(iii) In addition, studies to investigate serum markers of general tissue damage should be considered. Other determinations that should be carried out if the known properties of the test substance may, or are suspected to, affect related metabolic profiles include calcium, phosphate, fasting triglycerides and fasting glucose, specific hormones, methemoglobin and cholinesterase. These need to be identified on a case-by-case basis.
(iv) Overall, there is a need for a flexible approach, depending on the observed and/or expected effect with a given compound.
(v) If historical baseline data are inadequate, consideration should be given to determination of hematological and clinical biochemistry variables before dosing commences.
(11) Pathology—(i) Gross necropsy. (A) All adult animals in the study must be subjected to a full, detailed gross necropsy which includes careful examination of the external surface of the body, all orifices, and the cranial, thoracic and abdominal cavities and their contents. Special attention should be paid to the organs of the reproductive system. The number of implantation sites should be recorded. Corpora lutea should be counted.
(B) The testes and epididymides of all adult males should be weighed and the ovaries, testes, epididymides, accessory sex organs, and all organs showing macroscopic lesions of all adult animals, should be preserved.
(C) In addition, for five adult males and females, randomly selected from each group, the liver, kidneys, adrenals, thymus, spleen, brain and heart should be trimmed of any adherent tissue, as appropriate and their wet weight taken as soon as possible after dissection to avoid drying. Of the selected males and females, the following tissues should also be preserved in the most appropriate fixation medium for both the type of tissue and the intended subsequent histopathological examination: all gross lesions, brain (representative regions including cerebrum, cerebellum and pons), spinal cord, stomach, small and large intestines (including Peyer's patches), liver, kidneys, adrenals, spleen, heart, thymus, thyroid, trachea and lungs (preserved by inflation with fixative and then immersion), uterus, urinary bladder, lymph nodes (preferably 1 lymph node covering the route of administration and another one distant from the route of administration to cover systemic effects), peripheral nerve (sciatic or tibial) preferably in close proximity to the muscle, and a section of bone marrow (or, alternatively, a fresh mounted marrow aspirate).
(D) Formalin fixation is not recommended for routine examination of testes and epididymides. An acceptable method is the use of Bouin's fixative for these tissues. The clinical and other findings may suggest the need to examine additional tissues. Also, any organs considered likely to be target organs based on the known properties of the test substance should be preserved.
(E) Dead pups and pups sacrificed at day 4 post-partum, or shortly thereafter, should, at least, be carefully examined externally for gross abnormalities.
(ii) Histopathology. (A) Full histopathology should be conducted on the preserved organs and tissues of the selected animals in the control and high dose groups and all gross lesions. These examinations should be extended to animals of other dosage groups if treatment-related changes are observed in the high dose group.
(B) Detailed testicular histopathological examination (e.g., using Bouin's fixative, paraffin embedding and transverse sections of 4-5 ±m thickness) should be conducted with special emphasis on stages of spermatogenesis and histopathology interstitial testicular cell structure. The evaluation should identify treatment-related effects such as retained spermatids, missing germ cell layers or types, multinucleated giant cells or sloughing of spermatogenic cells into the lumen (the specifications for the evaluation are discussed in paragraph (g)(2) of this section). Examination of the intact epididymis should include the caput, corpus, and cauda, which can be accomplished by evaluation of a longitudinal section. The epididymis should be evaluated for leukocyte infiltration, change in prevalence of cell types, aberrant cell types, and phagocytosis of sperm. Periodic acid-Schiff (PAS) and hematoxylin staining may be used for examination of the male reproductive organs. Histopathological examination of the ovary should detect qualitative depletion of the primordial follicle population.
(C) When a satellite group is used, histopathology should be performed on Start Printed Page 78797tissues and organs identified as showing effects in the treated groups.
(g) Data and reporting—(1) Data. Individual animal data should be provided. Additionally, all data should be summarised in tabular form, showing for each test group the number of animals at the start of the test, the number of animals found dead during the test or sacrificed for humane reasons, the time of any death or humane sacrifice, the number of fertile animals, the number of pregnant females, the number of animals showing signs of toxicity, a description of the signs of toxicity observed, including time of onset, duration, and severity of any toxic effects, the types of histopathological changes, and all relevant litter data.
(2) Evaluation of results. (i) The findings of this toxicity study should be evaluated in terms of the observed effects, necropsy and microscopic findings. The evaluation will include the relationship between the dose of the test substance and the presence or absence, incidence and severity of abnormalities, including gross lesions, identified target organs, infertility, clinical abnormalities, affected reproductive and litter performance, body weight changes, effects on mortality and any other toxic effects.
(ii) Because of the short period of treatment of the male, the histopathology of the testes and epididymides must be considered along with the fertility data, when assessing male reproduction effects. The use of historic control data on reproduction/development (e.g. for litter size) where available may also be useful as an aid to the interpretation of the study.
(iii) When possible, numerical results should be evaluated by an appropriate and general acceptable statistical method. The statistical methods should be selected during the design of the study. Due to the limited dimensions of the study, statistical analysis in the form of tests for “significance” are of limited value for many endpoints, especially reproductive endpoints. Some of the most widely used methods, especially parametric tests for measures of central tendency, are inappropriate. If statistical analyses are used then the method chosen should be appropriate for the distribution of the variable examined and be selected prior to the start of the study.
(3) Test report. The test report must include the following information:
(i) Test substance:
(A) Physical nature and, where relevant, physicochemical properties.
(B) Identification data.
(ii) Vehicle (if appropriate): Justification for choice of vehicle, if other than water.
(iii) Test animals:
(A) Species/strain used.
(B) Number, age and sex of animals.
(C) Source, housing conditions, diet, etc.
(D) Individual weights of animals at the start of the test.
(iv) Test conditions:
(A) Rationale for dose level selection.
(B) Details of test substance formulation/diet preparation, achieved concentration, stability and homogeneity of the preparation.
(C) Details of the administration of the test substance.
(D) Conversion from diet/drinking water test substance concentration (parts per mission (ppm)) to the actual dose (mg/kg body weight/day), if applicable.
(E) Details of food and water quality.
(v) Results (toxic response data by sex and dose):
(A) Time of death during the study or whether animals survived to termination.
(B) Nature, severity and duration of clinical observations (whether reversible or not).
(C) Body weight/body weight change data.
(D) Food consumption and water consumption, if applicable.
(E) Sensory activity, grip strength and motor activity assessments.
(F) Hematological tests with relevant baseline values,
(G) Clinical biochemistry tests with relevant baseline values.
(H) Effects on reproduction, including information on mating/precoital interval, fertility, fecundity and gestation duration.
(I) Effects on offspring, including number of pups born (live and dead), sex ratio, postnatal growth (pup weights) and survival (litter size), gross abnormalities and clinical observations during lactation.
(J) Body weight at termination and organ weight data for the parental animals.
(K) Necropsy data, including number of implantations and number of corpora lutea.
(L) Calculations of pre- and postimplantation loss.
(M) Detailed description of histopathological findings.
(N) Statistical treatment of results, where appropriate.
(vi) Discussion of results.
(vii) Conclusions.
(h) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., NW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Mitsumori, K., Kodama, Y., Uchida, O., Takada, K., Saito, M. Naito, K., Tanaka, S., Kurokawa, Y., Usami, M., Kawashima, K., Yasuhara, K., Toyoda, K., Onodera, H., Furukawa, F., Takahashi, M. and Hayashi, Y., (1994). Confirmation Study, Using Nitro-Benzene, of the Combined Repeat Dose and Reproductive/ Developmental Toxicity Test Protocol Proposed by the Organization for Economic Cooperation and Development (OECD). Journal of Toxicology and Science, 19:141-149.
(2) Tanaka, S., Kawashima, K., Naito, K., Usami, M., Nakadate, M., Imaida, K., Takahashi, M., Hayashi, Y., Kurokawa, Y. and Tobe, M. (1992). Combined Repeat Dose and Reproductive/Developmental Toxicity Screening Test (OECD): Familiarization Using Cyclophosphamide. Fundamental and Applied Toxicology, 18:89-95.
(3) Tupper D.E., Wallace R.B. (1980). Utility of the Neurologic Examination in Rats. Acta Neurobiological Exposure, 40:999-1003.
(4) Gad S.C. (1982). A Neuromuscular Screen for Use in Industrial Toxicology. Journal of Toxicology and Environmental Health, 9:691-704.
(5) Moser V.C., McDaniel K.M., Phillips P.M. (1991). Rat Strain and Stock Comparisons Using a Functional Observational Battery: Baseline Values and Effects of Amitraz. Toxicology and Applied Pharmacology, 108:267-283.
(6) Meyer O.A., Tilson H.A., Byrd W.C., Riley M.T. (1979). A Method for the Routine Assessment of Fore- and Hindlimb Grip Strength of Rats and Mice. Neurobehavorial Toxicology, 1:233-236.
(7) Crofton K.M., Howard J.L., Moser V.C., Gill M.W., Reiter L.W., Tilson H.A., MacPhail R.C. (1991). Interlaboratory Comparison of Motor Activity Experiments: Implication for Neurotoxicological Assessments. Neurotoxicology and Teratology 13:599-609.
TSCA chronic toxicity.(a) Scope— (1) Applicability. This section is intended to meet the testing requirement of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides and Toxic Substances (OPPTS) harmonized test guideline 870.4100 Start Printed Page 78798(August 1998, final guidelines). This source is available at the address in paragraph (h) of this section
(b) Purpose. The objective of a chronic toxicity study is to determine the effects of a substance in a mammalian species following prolonged and repeated exposure. A chronic toxicity study should generate data from which to identify the majority of chronic effects and to define long-term dose-response relationships. The design and conduct of chronic toxicity tests should allow for the detection of general toxic effects, including neurological, physiological, biochemical, and hematological effects and exposure-related morphological (pathological) effects.
(c) Definitions. The definitions in section 3 of TSCA and in 40 CFR Part 792—Good Laboratory Practice Standards apply to this section. The following definitions also apply to this section.
Chronic toxicity is the adverse effects occurring as a result of the repeated daily exposure of experimental animals to a chemical by the oral, dermal, or inhalation routes of exposure.
Cumulative toxicity is the adverse effects of repeated doses occurring as a result of prolonged action on, or increased concentration of, the administered test substance or its metabolites in susceptible tissue.
Dose in a chronic toxicity study is the amount of test substance administered daily via the oral, dermal or inhalation routes for a period of at least 12 months. Dose is expressed as weight of the test substance (grams, milligrams) per unit body weight of test animal (milligram per kilogram), or as weight of the test substance in parts per million (ppm) in food or drinking water per day. For inhalation exposure, dose is expressed as weight of the test substance per unit volume of air (milligrams per liter) or as parts per million per day. For dermal exposure, dose is expressed as weight of the test substance (grams, milligrams) per unit body weight of the test animal (milligrams per kilogram) or as weight of the substance per unit of surface area (milligrams per square centimeter) per day.
No-observed-effects level (NOEL) is the maximum dose used in a study which produces no adverse effects. The NOEL is usually expressed in terms of the weight of a test substance given daily per unit weight of test animal (milligrams per kilogram per day).
Target organ is any organ of a test animal showing evidence of an effect induced by a test substance.
(d) Limit test. If a test at one dose level of at least 1,000 mg/kg body weight (expected human exposure may indicate the need for a higher dose level), using the procedures described for this study, produces no observable toxic effects and if toxicity would not be expected based upon data of structurally related compounds, a full study using three dose levels might not be necessary.
(e) Test procedures— (1) Animal selection— (i) Species and strain. Testing should be performed with two mammalian species, one a rodent and the other a nonrodent. The rat is the preferred rodent species. Commonly used laboratory strains must be employed.
(ii) Age/weight. (A) Testing must be started with young healthy animals as soon as possible after weaning and acclimatization.
(B) Dosing of rodents should generally begin no later than 8 weeks of age.
(C) Dosing of non-rodents should begin between 4 and 6 months of age and in no case later than 9 months of age.
(D) At commencement of the study, the weight variation of animals used should be within 20% of the mean weight for each sex.
(E) Studies using prenatal or neonatal animals may be recommended under special conditions.
(iii) Sex. (A) Equal numbers of animals of each sex should be used at each dose level.
(B) Females should be nulliparous and nonpregnant.
(iv) Numbers. (A) For rodents, at least 40 animals (20 males and 20 females) and for nonrodents at least 8 animals (4 females and 4 males) should be used at each dose level and concurrent control group.
(B) If interim sacrifices are planned, the number should be increased by the number of animals scheduled to be sacrificed during the course of the study.
(C) The number of animals at the termination of the study must be adequate for a meaningful and valid statistical evaluation of chronic effects. The Agency must be notified if excessive early deaths or other problems are encountered that might compromise the integrity of the study.
(D) To avoid bias, the use of adequate randomization procedures for the proper allocation of animals to test and control groups is required.
(E) Each animal should be assigned a unique identification number. Dead animals, their preserved organs and tissues, and microscopic slides should be identified by reference to the unique numbers assigned.
(v) Husbandry. (A) Rodents may be group-caged by sex, but the number of animals per cage must not interfere with clear observation of each animal. The biological properties of the test substance or toxic effects (e.g., morbidity, excitability) may indicate a need for individual caging. Rodents should be housed individually in dermal studies and during exposure in inhalation studies. Caging should be appropriate to the nonrodent species.
(B) The temperature of the experimental animal rooms should be at 22 ± 3 °C.
(C) The relative humidity of the experimental animal rooms should be 50 ± 20%.
(D) Where lighting is artificial, the sequence should be 12 hours light/12 hours dark.
(E) Control and test animals should be fed from the same batch and lot. The feed should be analyzed to assure adequacy of nutritional requirements of the species tested and for impurities that might influence the outcome of the test. Animals should be fed and watered ad libitum with food replaced at least weekly.
(F) The study should not be initiated until animals have been allowed a period of acclimatization/quarantine to environmental conditions, nor should animals from outside sources be placed on test without an adequate period of quarantine. An acclimation period of at least 5 days is recommended.
(2) Control and test substances. (i) Where necessary, the test substance is dissolved or suspended in a suitable vehicle. If a vehicle or diluent is needed it should not elicit toxic effects itself nor substantially alter the chemical or toxicological properties of the test substance. It is recommended that wherever possible the use of an aqueous solution be the first choice, followed by consideration of solution in oil, and finally, solution in other vehicles.
(ii) One lot of the test substance should be used, if possible, throughout the duration of the study, and the research sample should be stored under conditions that maintain its purity and stability. Prior to the initiation of the study, there should be a characterization of the test substance, including the purity of the test compound, and, if technically feasible, the names and quantities of contaminants and impurities.
(iii) If the test or control substance is to be incorporated into feed or another vehicle, the period during which the test substance is stable in such a mixture should be determined prior to the initiation of the study. Its homogeneity and concentration should be determined prior to the initiation of the study and periodically during the study. Statistically randomized samples Start Printed Page 78799of the mixture should be analyzed to ensure that proper mixing, formulation, and storage procedures are being followed, and that the appropriate concentration of the test or control substance is contained in the mixture.
(3) Control groups. A concurrent control group is required. This group should be an untreated or sham-treated control group or, if a vehicle is used in administering the test substance, a vehicle control group. If the toxic properties of the vehicle are not known or cannot be made available, both untreated and vehicle control groups are required.
(4) Satellite group. A satellite group of 40 animals (20 animals per sex) for rodents and 8 animals (4 animals per sex) for nonrodents may be treated with the high-dose level for 12 months and observed for reversibility, persistence, or delayed occurrence of toxic effects for a post-treatment of appropriate length, normally not less than 28 days. In addition, a control group of 40 animals (20 animals per sex) for rodents and 8 animals (4 animals per sex) for nonrodents should be added to the satellite study.
(5) Dose levels and dose selections. (i) In chronic toxicity tests, it is desirable to determine a dose-response relationship as well as a NOEL. Therefore, at least three dose levels with a control group and, where appropriate, a vehicle control (corresponding to the concentration of the vehicle at the highest exposure level) should be used. Dose levels should be spaced to produce a gradation of effects. A rationale must be provided for the doses selected.
(ii) The highest-dose level should elicit signs of toxicity without substantially altering the normal life span of the animal. The highest dose should be determined based on the findings from a 90-day study to ensure that the dose used is adequate to assess the chronic toxicity of the test substance. Thus, the selection of the highest dose to be tested is dependent upon changes observed in several toxicological parameters in subchronic studies. The highest dose tested need not exceed 1,000 mg/kg/day. If dermal application of the test substance produces severe skin irritation, then it may be necessary either to terminate the study and choose a lower high-dose level or to reduce the dose level. Gross criteria for defining severe irritation would include ulcers, fissures, exudate/crust(eschar), dead tissue, or anything leading to destruction of the functional integrity of the epidermis (e.g. caking, open sores, fissuring, eschar). Histological criteria for defining severe irritation would include follicular and interfollicular crust, microulcer, mild/moderate degeneration/necrosis, moderate/marked epidermal edema, marked dermal edema, and marked inflammation.
(iii) The intermediate dose levels should be spaced to produce a gradation of toxic effects.
(iv) The lowest-dose level should produce no evidence of toxicity.
(6) Administration of the test substance. The three main routes of administration are oral, dermal, and inhalation. The choice of the route of administration depends upon the physical and chemical characteristics of the test substance and the form typifying exposure in humans.
(i) Oral studies. Ideally, the animals should be dosed by gavage or with capsules on a 7-day per week basis for a period of at least 12 months. However, based primarily on practical considerations, dosing by gavage or capsules on a 5-day per week schedule is acceptable. If the test substance is administered via in the drinking water or mixed in the diet, exposure should be on a 7-day per week basis.
(ii) Dermal studies. (A) Preparation of animal skin. Shortly before testing, fur should be clipped from not less than 10% of the body surface area for application of the test substance. In order to dose approximately 10% of the body surface, the area starting at the scapulae (shoulders) to the wing of the ileum (hipbone) and half way down the flank on each side of the animal should be shaved. Shaving should be carried out approximately 24 hours before dosing. Repeated clipping or shaving is usually needed at approximately weekly intervals. When clipping or shaving the fur, care should be taken to avoid abrading the skin which could alter its permeability.
(B) Preparation of test substance. Liquid test substances are generally used undiluted, except as indicated in paragraph (e)(5)(ii) of this section. Solids should be pulverized when possible. The substance should be moistened sufficiently with water or, when necessary, with a suitable vehicle to ensure good contact with the skin. When a vehicle is used, the influence of the vehicle on toxicity of, and penetration of the skin by, the test substance should be taken into account.The volume of application should be kept constant, e.g., less than 100 mL for the mouse and less than 300 mL for the rat. Different concentrations of test solution should be prepared for different dose levels.
(C) Administration of test substance. The duration of exposure should be at least for 12 months. Ideally, the animals should be treated with test substance for at least 6 hours per day on a 7-day per week basis. However, based on practical considerations, application on a 5-day per week basis is acceptable. Dosing should be conducted at approximately the same time each day. The test substance should be applied uniformly over the treatment site. The surface area covered may be less for highly toxic substances. As much of the area should be covered with as thin and uniform a film as possible. For rats, the test substance may be held in contact with the skin with a porous gauze dressing and nonirritating tape if necessary. The test site should be further covered in a suitable manner to retain the gauze dressing plus test substance and to ensure that the animals cannot ingest the test substance. The application site should not be covered when the mouse is the species of choice. The test substance may be wiped from the skin after the six-hour exposure period to prevent ingestion.
(iii) Inhalation studies. (A) The animals should be exposed to the test substance for 6 hours per day on a 7-day per week basis, for a period of at least 12 months. However, based primarily on practical considerations, exposure for 6 hours per day on a 5-day per week basis is acceptable.
(B) The animals should be tested in dynamic inhalation equipment designed to sustain a minimum air flow of 10 air changes per hour, an adequate oxygen content of at least 19%, and uniform conditions throughout the exposure chamber. Maintenance of slight negative pressure inside the chamber will prevent leakage of the test substance into surrounding areas. It is not normally necessary to measure chamber oxygen concentration if airflow is adequate.
(C) The selection of a dynamic inhalation chamber should be appropriate for the test substance and test system. When a whole body chamber is used, individual housing must be used to minimize crowding of the test animals and maximize their exposure to the test substance. To ensure stability of a chamber atmosphere, the total volume occupied by the test animals should not exceed 5% of the volume of the test chamber. It is recommended, but not required, that nose-only or head-only exposure be used for aerosol studies in order to minimize oral exposures due to animals licking compound off their fur. The animals should be acclimated and heat stress minimized.
(D) The temperature at which the test is performed should be maintained at 22 ± 2 °C. The relative humidity should be Start Printed Page 78800maintained between 40-60%, but in certain instances (e.g., use of water vehicle) this may not be practicable.
(E) The rate of air flow should be monitored continuously but recorded at least three times during the exposure.
(F) Temperature and humidity should be monitored continuously but should be recorded at least every 30 min.
(G) The actual concentrations of the test substance should be measured in the breathing zone. During the exposure period, the actual concentrations of the test substance should be held as constant as practicable, monitored continuously or intermittently depending on the method of analysis. Chamber concentration may be measured using gravimetric or analytical methods, as appropriate. If trial run measurements are reasonably consistent (± 10% for liquid aerosol, gas, or vapor; ± 20% for dry aerosol), then two measurements should be sufficient. If measurements are not consistent, three to four measurements should be taken. If there is some difficulty measuring chamber analytical concentration due to precipitation, nonhomogeneous mixtures, volatile components, or other factors, additional analysis of inert components may be necessary.
(H) During the development of the generating system, particle size analysis should be performed to establish the stability of aerosol concentrations with respect to particle size. The mass median aerodynamic diameter (MMAD) particle size range should be between 1-3 mm. The particle size of hygroscopic materials should be small enough when dry to assure that the size of the swollen particle will still be within the 1-3 mm range. Measurements of aerodynamic particle size in the animal's breathing zone should be measured during a trial run. If MMAD values for each exposure level are within 10% of each other, then two measurements during the exposures should be sufficient. If pretest measurements are not within 10% of each other, three to four measurements should be taken.
(I) Feed should be withheld during exposure. Water may also be withheld during exposure.
(7) Observation period. (i) Animals should be observed for a period of at least 12 months.
(ii) Animals in a satellite group (if used) scheduled for follow-up observations should be kept for at least 28 days further without treatment to detect recovery from, or persistence of, toxic effects.
(8) Observation of animals. (i) Observations should be made at least twice each day for morbidity and mortality. Appropriate actions should be taken to minimize loss of animals to the study (e.g., necropsy or refrigeration of those animals found dead and isolation or sacrifice of weak or moribund animals). General clinical observations should be made at least once a day, preferably at the same time each day, taking into consideration the peak period of anticipated effects after dosing. The clinical condition of the animal should be recorded.
(ii) A careful clinical examination should be made at least once prior to the initiation of treatment (to allow for within subject comparisons) and once weekly during treatment in all animals. These observations should be made outside the home cage, preferably in a standard arena, and at similar times on each occasion. Effort should be made to ensure that variations in the observation conditions are minimal. Observations should be detailed and carefully recorded, preferably using scoring systems, explicitly defined by the testing laboratory. Signs noted should include, but not be limited to, changes in skin, fur, eyes, mucous membranes, occurrence of secretions and excretions and autonomic activity (e.g., lacrimation, piloerection, pupil size, unusual respiratory pattern). Changes in gait, posture and response to handling as well as the presence of clonic or tonic movements, stereotypies (e.g., excessive grooming, repetitive circling) or bizarre behavior (e.g., self-mutilation, walking backwards) should be recorded.
(iii) Once, near the end of the first year of the exposure period and in any case not earlier than in month 11, assessment of motor activity, grip strength, and sensory reactivity to stimuli of different types (e.g., visual, auditory, and proprioceptive stimuli) should be conducted in rodents. Further details of the procedures that could be followed are described in the references listed under paragraphs (h)(2), (h)(7), (h)(8), and (h)(11) of this section.
(iv) Functional observations conducted towards the end of the study may be omitted when data on functional observations are available from other studies and the daily clinical observations did not reveal any functional deficits.
(v) Exceptionally, functional observations may be omitted for groups that otherwise reveal signs of toxicity to an extent that would significantly interfere with functional test performance.
(vi) Body weights should be recorded individually for all animals once prior to the administration of the test substance, once a week during the first 13 weeks of study and at least once every 4 weeks thereafter, unless signs of clinical toxicity suggest more frequent weighing to facilitate monitoring of health status.
(vii) Measurements of feed consumption should be determined weekly during the first 13 weeks of the study and at approximately monthly intervals thereafter unless health status or body weight changes dictate otherwise. Measurements of water consumption should be determined at the same intervals if the test substance is administered in the drinking water.
(viii) Moribund animals should be removed and sacrificed when noticed and the time of death should be recorded as precisely as possible. All survivors should be sacrificed at the end of the study period.
(9) Clinical pathology. Hematology, clinical chemistry, and urinalysis should be performed on 10 rats per sex per group, and on all nonrodents. In rodents, the parameters should be examined at approximately 6 month intervals during the conduct of the study and at termination. If possible, these collections should be from the same animals at each interval. In nonrodents, the parameters should be examined once or twice prior to initiation of treatment, at 6-month intervals during the conduct of the study, and at termination. If hematological and biochemical effects were seen in the subchronic study, testing should also be performed at 3 months. Overnight fasting of animals prior to blood sampling is recommended.
(i) Hematology. The recommended parameters are red blood cell count, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration, white blood cell count, differential leukocyte count, platelet count, and a measure of clotting potential, such as prothrombin time or activated partial thromboplastin time.
(ii) Clinical chemistry. (A) Parameters which are considered appropriate to all studies are electrolyte balance, carbohydrate metabolism, and liver and kidney function. The selection of specific tests will be influenced by observations on the mode of action of the substance and signs of clinical toxicity.
(B) The recommended clinical chemistry determinations are potassium, sodium, calcium (nonrodent), phosphorus (nonrodent), chloride (nonrodent), glucose, total cholesterol, urea nitrogen, creatinine, total protein, total bilirubin (nonrodent), and albumin. More than two hepatic Start Printed Page 78801enzymes, (such as alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, sorbitol dehydrogenase, or gamma glutamyl transpeptidase) should also be measured. Measurements of additional enzymes (of hepatic or other origin) and bile acids, may also be useful.
(C) If a test chemical has an effect on the hematopoietic system, reticulocyte counts and bone marrow cytology may be indicated.
(D) Other determinations that should be carried out if the test chemical is known or suspected of affecting related measures include calcium, phosphorus, fasting triglycerides, hormones, methemoglobin, and cholinesterases.
(iii) Urinalysis. Urinalysis for rodents should be performed at the end of the study using timed urine collection. Urinalysis for nonrodents should be performed prior to treatment, midway through treatment and at the end of the study using timed urine collection. Urinalysis determinations include: appearance, volume, osmolality or specific gravity, pH, protein, glucose, and blood/blood cells.
(10) Ophthalmological examination. Examinations should be made of all animals using an ophthalmoscope or equivalent device prior to the administration of the test substance and at termination of the study on 10 rats of each sex in the high-dose and control groups and preferably in all nonrodents, but at least the control and high-dose groups should be examined. If changes in eyes are detected, all animals should be examined.
(11) Gross necropsy. (i) All animals should be subjected to a full gross necropsy which includes examination of the external surface of the body, all orifices, and the cranial, thoracic and abdominal cavities and their contents.
(ii) At least the liver, kidneys, adrenals, testes, epididymides, ovaries, uterus, nonrodent thyroid (with parathyroid), spleen, brain, and heart should be weighed wet as soon as possible after dissection to avoid drying. The lungs should be weighed if the test substance is administered by the inhalation route.
(iii) The following organs and tissues, or representative samples thereof, should be preserved in a suitable medium for possible future histopathological examination:
(A) Digestive system—salivary glands, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, liver, pancreas, gallbladder (when present).
(B) Nervous system—brain (multiple sections, including cerebrum, cerebellum and medulla/pons), pituitary, peripheral nerve (sciatic or tibial, preferably in close proximity to the muscle), spinal cord (three levels, cervical, mid-thoracic and lumbar), eyes (retina, optic nerve).
(C) Glandular system—adrenals, parathyroid, thyroid.
(D) Respiratory system—trachea, lungs, pharynx, larynx, nose.
(E) Cardiovascular/hematopoietic system—aorta, heart, bone marrow (and/or fresh aspirate), lymph nodes (preferably one lymph node covering the route of administration and another one distant from the route of administration to cover systemic effects), spleen.
(F) Urogenital system—kidneys, urinary bladder, prostate, testes, epididymides, seminal vesicle(s), uterus, ovaries, female mammary gland.
(G) Other—all gross lesions and masses, skin.
(iv) In inhalation studies, the entire respiratory tract, including nose, pharynx, larynx, and paranasal sinuses should be examined and preserved. In dermal studies, skin from treated and adjacent control skin sites should be examined and preserved.
(v) Inflation of lungs and urinary bladder with a fixative is the optimal method for preservation of these tissues. The proper inflation and fixation of the lungs in inhalation studies is considered essential for appropriate and valid histopathological examination.
(vi) Information from clinical pathology and other in-life data should be considered before microscopic examination, since they may provide significant guidance to the pathologist.
(12) Histopathology. (i) The following histopathology should be performed:
(A) Full histopathology on the organs and tissues (listed under paragraph (e)(11)(iii) of this section) of all rodents and nonrodents in the control and high-dose groups, and all rodents and nonrodents that died or were sacrificed during the study. The examination should be extended to all animals in all dosage groups if treatment-related changes are observed in the high-dose group.
(B) All gross lesions in all animals.
(C) Target tissues in all animals.
(ii) If the results show substantial alteration of the animal's normal life span, or other effects that might compromise the significance of the data, the next lower levels should be examined fully as described in paragraph (e)(12)(i) of this section.
(iii) An attempt should be made to correlate gross observations with microscopic findings.
(iv) Tissues and organs designated for microscopic examination should be fixed in 10% buffered formalin or a recognized suitable fixative as soon as necropsy is performed and no less than 48 hours prior to trimming.
(f) Data and reporting— (1) Treatment of results. (i) Data should be summarized in tabular form, showing for each test group the number of animals at the start of the test, the number of animals showing lesions, the types of lesions and the percentage of animals displaying each type of lesion.
(ii) When applicable, all observed results (quantitative and qualitative) should be evaluated by an appropriate statistical method. Any generally accepted statistical methods may be used; the statistical methods including significance criteria should be selected during the design of the study.
(2) Evaluation of study results. The findings of a chronic toxicity study should be evaluated in conjunction with the findings of preceding studies and considered in terms of the toxic effects as well as the necropsy and histopathological findings. The evaluation will include the relationship between the dose of the test substance and the presence, incidence, and severity of abnormalities (including behavioral and clinical abnormalities), gross lesions, identified target organs, body weight changes, effects on mortality and any other general or specific toxic effects.
(3) Test report. In addition to the reporting requirements specified under EPA Good Laboratory Practice Standards at 40 CFR part 792, subpart J, the following specific information must be reported:
(i) Test substance characterization should include:
(A) Chemical identification.
(B) Lot or batch number.
(C) Physical properties.
(D) Purity/impurities.
(ii) Identification and composition of any vehicle used.
(iii) Test system should contain data on:
(A) Species and strain of animals used and rationale for selection if other than that recommended.
(B) Age including body weight data and sex.
(C) Test environment including cage conditions, ambient temperature, humidity, and light/dark periods.
(D) Identification of animal diet.
(E) Acclimation period.
(iv) Test procedure should include the following data:
(A) Method of randomization used.
(B) Full description of experimental design and procedure.
(C) Dose regimen including levels, methods, and volume. Start Printed Page 78802
(v) Test results.
(A) Group animal data. Tabulation of toxic response data by species, strain, sex and exposure level for:
(1) Number of animals exposed.
(2) Number of animals showing signs of toxicity.
(3) Number of animals dying.
(B) Individual animal data. Data should be presented as summary (group mean) as well as for individual animals.
(1) Time of death during the study or whether animals survived to termination.
(2) Time of observation of each abnormal sign and its subsequent course.
(3) Body weight data.
(4) Feed and water (if collected) consumption data.
(5) Achieved dose (mg/kg/day) as a time-weighted average if the test substance is administered in the diet or drinking water.
(6) Results of ophthalmological examinations.
(7) Results of hematological tests performed.
(8) Results of clinical chemistry tests performed.
(9) Urinalysis tests performed and results.
(10) Results of observations made.
(11) Necropsy findings, including absolute and relative (to body weight) organ weight data.
(12) Detailed description of all histopathological findings.
(13) Statistical treatment of results, where appropriate.
(vi) In addition, for inhalation studies the following should be reported:
(A) Test conditions. The following exposure conditions must be reported:
(1) Description of exposure apparatus including design, type, dimensions, source of air, system for generating particulate and aerosols, method of conditioning air, treatment of exhaust air and the method of housing the animals in a test chamber.
(2) The equipment for measuring temperature, humidity, and particulate aerosol concentrations and size should be described.
(B) Exposure data. These data should be tabulated and presented with mean values and a measure of variability (e.g., standard deviation) and should include:
(1) Airflow rates through the inhalation equipment.
(2) Temperature and humidity of air.
(3) Actual (analytical or gravimetric) concentration in the breathing zone.
(4) Nominal concentration (total amount of test substance fed into the inhalation equipment divided by volume of air).
(5) Particle size distribution, calculated MMAD, and geometric standard deviation.
(6) Explanation as to why the desired chamber concentration and/or particle size could not be achieved (if applicable) and the efforts taken to comply with this aspect of the guidelines.
(g) Quality control. A system should be developed and maintained to assure and document adequate performance of laboratory staff and equipment. The study must be conducted in compliance with 40 CFR Part 792—Good Laboratory Practice Standards.
(h) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., SW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Benitz, K.F. Measurement of Chronic Toxicity. Methods of Toxicology. Ed. G.E. Paget. Blackwell, Oxford. pp. 82-131 (1970).
(2) Crofton K.M., Howard J.L., Moser V.C., Gill M.W., Leiter L.W., Tilson H.A., MacPhail, R.C. Interlaboratory Comparison of Motor Activity Experiments: Implication for Neurotoxicological Assessments. Neurotoxicol. Teratol. 13, 599-609. (1991)
(3) D'Aguanno, W. Drug Safety Evaluation--Pre-Clinical Considerations. Industrial Pharmacology: Neuroleptic. Vol. I, Ed. S. Fielding and H. Lal. Futura, Mt. Kisco, NY. pp. 317-332 (1974).
(4) Fitzhugh, O.G. Chronic Oral Toxicity, Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics. The Association of Food and Drug Officials of the United States. pp. 36-45 (1959, 3rd Printing 1975).
(5) Gad S.C. A Neuromuscular Screen for Use in Industrial Toxicology. Journal of Toxicology and Environmental Health. 9, 691-704. (1982)
(6) Goldenthal, E.I. and D'Aguanno, W. Evaluation of Drugs, Appraisal of the Safety of Chemicals in Foods, Drugs, and Cosmetics. The Association of Food and Drug Officials of the United States. pp. 60-67 (1959, 3rd Printing 1975).
(7) Meyer O.A., Tilson H.A., Byrd W.C., Riley M.T. A Method for the Routine Assessment of Fore- and Hind-Limb Grip Strength of Rats and Mice. Neurobehav. Toxicol. 1, 233-236. (1979)
(8) Moser V.C., McDaniel K.M., Phillips P.M. Rat Strain and Stock Comparisons using a Functional Observational Battery: Baseline Values and Effects of Amitraz. Toxicol. Appl. Pharmacol. 108, 267-283 (1991)
(9) Organization for Economic Cooperation and Development. Guidelines for Testing of Chemicals, Section 4-Health Effects, Part 452 Chronic Toxicity Studies, Paris (1981).
(10) Page, N.P. Chronic Toxicity and Carcinogenicity Guidelines. Journal of Environmental Pathology and Toxicology. 11:161-182 (1977).
(11) Tupper, D.E., Wallace R.B. Utility of the Neurologic Examination in Rats. Acta. Neurobiol. Exp. 40, 999-1003 (1980).
(12) Weingand K., Brown G., Hall R. et al. (1996). Harmonization of Animal Clinical Pathology Testing in Toxicity and Safety Studies. Fundam. and Appl. Toxicol. 29:198-201.
TSCA combined chronic toxicity/carcinogenicity.(a) Scope. This section is intended to meet the testing requirements under section 4 of the Toxic Substances Control Act (TSCA). The objective of a combined chronic toxicity/carcinogenicity study is to determine the effects of a substance in a mammalian species following prolonged and repeated exposure. The application of this section should generate data which identify the majority of chronic and carcinogenicity effects and determine dose-response relationships. The design and conduct should allow for the detection of neoplastic effects and a determination of the carcinogenic potential as well as general toxicity, including neurological, physiological, biochemical, and hematological effects and exposure-related morphological (pathology) effects.
(b) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides, and Toxic Substances (OPPTS) harmonized test guideline 870.4300 (August 1998, final guideline). This source is available at the address in paragraph (h) of this section.
(c) Definitions. The following definitions apply to this section.
Carcinogenicity is the development of neoplastic lesions as a result of the repeated daily exposure of experimental animals to a chemical by the oral, dermal, or inhalation routes of exposure.
Chronic toxicity is the adverse effects occurring as a result of the repeated daily exposure of experimental animals to a chemical by the oral, dermal, or inhalation routes of exposure.
Cumulative toxicity is the adverse effects of repeated dose occurring as a result of prolonged action on, or Start Printed Page 78803increased concentration of, the administered test substance or its metabolites in susceptible tissues.
Dose in a combined chronic toxicity/carcinogenicity study is the amount of test substance administered via the oral, dermal, or inhalation routes for a period of up to 24 months. Dose is expressed as weight of the test substance per unit body weight of test animal (milligrams per kilogram), or as weight of the test substance in parts per million (ppm) in food or drinking water. When exposed via inhalation, dose is expressed as weight of the test substance per unit volume of air (milligrams per liter) or as parts per million per day. For dermal application, dose is expressed as weight of the test substance (grams, milligrams) per unit body weight of the test animal (milligrams per kilogram) or as weight of the substance per unit surface area (milligrams per square centimeter) per day.
No-observed-effects level (NOEL) is the maximum dose used in a study which produces no observed adverse effects. The NOEL is usually expressed in terms of the weight of a test substance given daily per unit weight of test animal (milligrams per kilogram per day).
Target organ is any organ of a test animal showing evidence of an effect induced by a test substance.
(d) Limit test. If a test at one dose level of at least 1,000 mg/kg body weight (expected human exposure may indicate the need for a higher dose level), using the procedures described for this study, produces no observable toxic effects or if toxic effects would not be expected based upon data of structurally related compounds, then a full study using three dose levels might not be necessary.
(e) Test procedures—(1) Animal selection—(i) Species and strain. Preliminary studies providing data on acute, subchronic, and metabolic responses should have been carried out to permit an appropriate choice of animals (species and strain). As discussed in other guidelines, the mouse and rat have been most widely used for assessment of carcinogenic potential, while the rat and dog have been most often studied for chronic toxicity. For the combined chronic toxicity/carcinogenicity study via the oral and inhalation routes, the rat is the species of choice and for the dermal route, the mouse is species of choice. If other species are used, the tester must provide justification/reasoning for their selection. The strain selected should be susceptible to the carcinogenic or toxic effect of the class of substances being tested, if known, and provided it does not have a spontaneous background incidence too high for meaningful assessment. Commonly used laboratory strains must be employed.
(ii) Age/weight. (A) Testing must be started with young healthy animals as soon as possible after weaning and acclimatization.
(B) Dosing should generally begin no later than 8 weeks of age.
(C) At commencement of the study, the weight variation of animals used must be within 20% of the mean weight for each sex.
(D) Studies using prenatal or neonatal animals may be recommended under special conditions.
(iii) Sex. (A) Equal numbers of animals of each sex must be used at each dose level.
(B) Females must be nulliparous and nonpregnant.
(iv) Numbers. (A) At least 100 rodents (50 males and 50 females) must be used at each dose level and concurrent control group. At least 20 additional rodents (10 males and 10 females) should be used for satellite dose groups and the satellite control group. The purpose of the satellite group is to allow for the evaluation of chronic toxicity after 12 months of exposure to the test substance.
(B) For a meaningful and valid statistical evaluation of long term exposure and for a valid interpretation of negative results, the number of animals in any group should not fall below 50% at 15 months in mice and 18 months in rats. Survival in any group should not fall below 25% at 18 months in mice and 24 months in rats.
(C) To avoid bias, the use of adequate randomization procedures for the proper allocation of animals to test and control groups is required.
(D) Each animal must be assigned a unique identification number. Dead animals (and their preserved organs) and tissues, and microscopic slides shall be identified by reference to the unique numbers assigned.
(v) Husbandry. (A) Animals may be group-caged by sex, but the number of animals per cage must not interfere with clear observation of each animal. The biological properties of the test substance or toxic effects (e.g., morbidity, excitability) may indicate a need for individual caging. Rodents should be housed individually in dermal studies and during exposure in inhalation studies.
(B) The temperature of the experimental animal rooms should be at 22 ± 3 °C.
(C) The relative humidity of the experimental animal rooms should be 50 ± 20%.
(D) Where lighting is artificial, the sequence should be 12 hours light/12 hours dark.
(E) Control and test animals should be fed from the same batch and lot. The feed should be analyzed to assure uniform distribution and adequacy of nutritional requirements of the species tested and for impurities that might influence the outcome of the test. Animals should be fed and watered ad libitum with food replaced at least weekly.
(F) The study should not be initiated until animals have been allowed a period of acclimatization/quarantine to environmental conditions, nor should animals from outside sources be placed on test without an adequate period of quarantine. An acclimation period of at least five days is recommended.
(2) Control and test substances. (i) Where necessary, the test substance is dissolved or suspended in a suitable vehicle. If a vehicle or diluent is needed, it should not elicit toxic effects itself nor substantially alter the chemical or toxicological properties of the test substance. It is recommended that wherever possible the usage of an aqueous solution be considered first, followed by consideration of a solution in oil, and finally solution in other vehicles.
(ii) One lot of the test substance should be used throughout the duration of the study if possible, and the research sample should be stored under conditions that maintain its purity and stability. Prior to the initiation of the study, there should be a characterization of the test substance, including the purity of the test compound, and, if possible, the name and quantities of contaminants and impurities.
(iii) If the test or control substance is to be incorporated into feed or another vehicle, the period during which the test substance is stable in such a mixture should be determined prior to the initiation of the study. Its homogeneity and concentration should be determined prior to the initiation of the study and periodically during the study. Statistically randomized samples of the mixture should be analyzed to ensure that proper mixing, formulation, and storage procedures are being followed, and that the appropriate concentration of the test or control substance is contained in the mixture.
(3) Control groups. A concurrent control group is required. This group should be an untreated or sham-treated control group or, if a vehicle is used in administering the test substance, a vehicle control group. If the toxic properties of the vehicle are not known or cannot be made available, both Start Printed Page 78804untreated and vehicle control groups are required.
(4) Dose levels and dose selection. (i) For risk assessment purposes, at least three dose levels must be used, in addition to the concurrent control group. Dose levels should be spaced to produce a gradation of effects. A rationale for the doses selected must be provided.
(ii) The highest dose level in rodents should elicit signs of toxicity without substantially altering the normal life span due to effects other than tumors. The highest dose should be determined based on the findings from a 90-day study to ensure that the dose used is adequate to assess the chronic toxicity and the carcinogenic potential of the test substance. Thus, the selection of the highest dose to be tested is dependent upon changes observed in several toxicological parameters in subchronic studies. The highest dose tested need not exceed 1,000 mg/kg/day.
(iii) The intermediate-dose levels should be spaced to produce a gradation of toxic effects.
(iv) The lowest-dose level should produce no evidence of toxicity.
(v) For skin carcinogenicity studies, when toxicity to the skin is a determining factor, the highest dose selected should not destroy the functional integrity of the skin, the intermediate doses should be a minimally irritating dose and the low dose should be the highest nonirritating dose.
(vi) The criteria for selecting the dose levels for skin carcinogenicity studies, based on gross and histopathologic dermal lesions, are as follows:
(A) Gross criteria for reaching the high dose:
(1) Erythema (moderate).
(2) Scaling.
(3) Edema (mild).
(4) Alopecia.
(5) Thickening.
(B) Histologic criteria for reaching the high dose:
(1) Epidermal hyperplasia.
(2) Epidermal hyperkeratosis.
(3) Epidermal parakeratosis.
(4) Adnexal atrophy/hyperplasia.
(5) Fibrosis.
(6) Spongiosis (minimal-mild).
(7) Epidermal edema (minimal-mild).
(8) Dermal edema (minimal-moderate).
(9) Inflammation (moderate).
(C) Gross criteria for exceeding the high dose:
(1) Ulcers-fissures, exudate/crust (eschar), nonviable (dead) tissues.
(2) Anything leading to destruction of the functional integrity of the epidermis (e.g., caking, fissuring, open sores, eschar).
(D) Histologic criteria for exceeding the high-dose:
(1) Crust (interfollicular and follicular).
(2) Microulcer.
(3) Degeneration/necrosis (mild to moderate).
(4) Epidermal edema (moderate to marked).
(5) Dermal edema (marked).
(6) Inflammation (marked).
(5) Administration of the test substance. The three main routes of administration are oral, dermal, and inhalation. The choice of the route of administration depends upon the physical and chemical characteristics of the test substance and the form typifying exposure in humans.
(i) Oral studies. If the test substance is administered by gavage, the animals are dosed with the test substance on a 7-day per week basis for a period of at least 18 months for mice and hamsters and 24 months for rats. However, based primarily on practical considerations, dosing by gavage on a 5-day per week basis is acceptable. If the test substance is administered in the drinking water or mixed in the diet, then exposure should be on a 7-day per week basis.
(ii) Dermal studies. (A) Preparation of animal skin. Shortly before testing, fur should be clipped from not less than 10% of the body surface area for application of the test substance. In order to dose approximately 10% of the body surface, the area starting at the scapulae (shoulders) to the wing of the ileum (hipbone) and half way down the flank on each side of the animal should be shaved. Shaving should be carried out approximately 24 hours before dosing. Repeated clipping or shaving is usually needed at approximately weekly intervals. When clipping or shaving the fur, care should be taken to avoid abrading the skin which could alter its permeability.
(B) Preparation of test substance. Liquid test substances are generally used undiluted, except as indicated in paragraph (e)(4)(vi) of this section. Solids should be pulverized when possible. The substance should be moistened sufficiently with water or, when necessary, with a suitable vehicle to ensure good contact with the skin. When a vehicle is used, the influence of the vehicle on toxicity of, and penetration of the skin by, the test substance should be taken into account.The volume of application should be kept constant, e.g., less than 100 mL for the mouse and less than 300 mL for the rat. Different concentrations of test solution should be prepared for different dose levels.
(C) Administration of test substance. The duration of exposure should be at least 18 months for mice and hamsters and 24 months for rats. Ideally, the animals should be treated with test substance for at least 6 hours per day on a 7-day per week basis. However, based on practical considerations, application on a 5-day per week basis is acceptable. Dosing should be conducted at approximately the same time each day. The test substance must be applied uniformly over the treatment site.The surface area covered may be less for highly toxic substances. As much of the area should be covered with as thin and uniform a film as possible. For rats, the test substance may be held in contact with the skin with a porous gauze dressing and nonirritating tape if necessary. The test site should be further covered in a suitable manner to retain the gauze dressing plus test substance and to ensure that the animals cannot ingest the test substance. The application site should not be covered when the mouse is the species of choice. The test substance may be wiped from the skin after the 6-hour exposure period to prevent ingestion.
(iii) Inhalation studies. (A) The animals should be exposed to the test substance, for 6 hours per day on a 7-day per week basis, for a period of at least 18 months in mice and 24 months in rats. However, based primarily on practical considerations, exposure for 6 hours per day on a 5-day per week basis is acceptable.
(B) The animals must be tested in dynamic inhalation equipment designed to sustain a minimum air flow of 10 air changes per hour, an adequate oxygen content of at least 19%, and uniform conditions throughout the exposure chamber. Maintenance of slight negative pressure inside the chamber will prevent leakage of the test substance into surrounding areas. It is not normally necessary to measure chamber oxygen concentration if airflow is adequate.
(C) The selection of a dynamic inhalation chamber should be appropriate for the test substance and test system. Where a whole body chamber is used, individual housing must be used to minimize crowding of the test animals and maximize their exposure to the test substance. To ensure stability of a chamber atmosphere, the total volume occupied by the test animals shall not exceed 5% of the volume of the test chamber. It is recommended, but not required, that nose-only or head-only exposure be used for aerosol studies in order to minimize oral exposures due to animals licking compound off their fur. The Start Printed Page 78805animals should be acclimated and heat stress minimized.
(D) The temperature at which the test is performed should be maintained at 22 ± 2 °C. The relative humidity should be maintained between 40 to 60%, but in certain instances (e.g., tests of aerosols, use of water vehicle) this may not be practicable.
(E) The rate of air flow must be monitored continuously but recorded at least three times during the exposure.
(F) Temperature and humidity must be monitored continuously but should be recorded at least every 30 minutes.
(G) The actual concentrations of the test substance must be measured in the animal's breathing zone. During the exposure period, the actual concentrations of the test substance must be held as constant as practicable and monitored continuously or intermittently depending on the method of analysis. Chamber concentration may be measured using gravimetric or analytical methods as appropriate. If trial run measurements are reasonably consistent (± 10% for liquid aerosol, gas, or vapor; ± 20% for dry aerosol), then two measurements should be sufficient. If measurements are not consistent, three to four measurements should be taken. If there is some difficulty in measuring chamber analytical concentration due to precipitation, nonhomogeneous mixtures, volatile components, or other factors, additional analyses of inert components may be necessary.
(H) During the development of the generating system, particle size analysis must be performed to establish the stability of aerosol concentrations with respect to particle size. The mass median aerodynamic diameter (MMAD) particle size range should be between 1-3 mm. The particle size of hygroscopic materials should be small enough when dry to assure that the size of the swollen particle will still be within the 1-3 mm range. Measurements of aerodynamic particle size in the animal's breathing zone should be measured during a trial run. If MMAD values for each exposure level are within 10% of each other, then two measurements during the exposures should be sufficient. If pretest measurements are not within 10% of each other, three to four measurements should be taken.
(I) Feed must be withheld during exposure. Water may also be withheld during exposure.
(J) When the physical and chemical properties of the test substance show a low flash point or the test substance is otherwise known or thought to be explosive, care must be taken to avoid exposure level concentrations that could result in an exposure chamber explosion during the test.
(6) Observation period. (i) This time period must not be less than 24 months for rats and 18 months for mice, and ordinarily not longer than 30 months for rats and 24 months for mice. For longer time periods, and where any other species are used, consultation with the Agency in regard to the duration of the study is advised.
(ii) Animals in a satellite group to assess chronic toxicity should be observed for 12 months.
(7) Observation of animals. (i) Observations must be made at least twice each day for morbidity and mortality. Appropriate actions should be taken to minimize loss of animals to the study (e.g., necropsy or refrigeration of those animals found dead and isolation or sacrifice of weak or moribund animals). General clinical observations shall be made at least once a day, preferably at the same time each day, taking into consideration the peak period of anticipated effects after dosing. The clinical condition of the animal should be recorded.
(ii) A careful clinical examination must be made at least once weekly. Observations should be detailed and carefully recorded, preferably using explicity defined scales. Observations should include, but not be limited to, evaluation of skin and fur, eyes and mucous membranes, respiratory and circulatory effects, autonomic effects such as salivation, central nervous system effects, including tremors and convulsions, changes in the level of activity, gait and posture, reactivity to handling or sensory stimuli, altered strength, and stereotypes or bizarre behavior (e.g., self-mutilation, walking backwards).
(iii) Signs of toxicity should be recorded as they are observed including the time of onset, degree and duration.
(iv) Body weights must be recorded individually for all animals once prior to administration of the test substance, once a week during the first 13 weeks of the study and at least once every 4 weeks thereafter unless signs of clinical toxicity suggest more frequent weighing to facilitate monitoring of health status.
(v) Measurements of feed consumption should be determined weekly during the first 13 weeks of the study and then at approximately monthly intervals unless health status or body weight changes dictate otherwise. Measurements of water consumption should be determined at the same intervals if the test material is administered in drinking water.
(vi) Moribund animals must be removed and sacrificed when noticed and the time of death should be recorded as precisely as possible. At the end of the study period, all survivors must be sacrificed. Animals in the satellite group must be sacrificed after 12 months of exposure to the test substance (interim sacrifice).
(8) Clinical pathology. Hematology, clinical chemistry and urinalyses must be performed from 10 animals per sex per group. The parameters should be examined at approximately 6 month intervals during the first 12 months of the study. If possible, these collections should be from the same animals at each interval. If hematological and biochemical effects are seen in the subchronic study, testing shall also be performed at 3 months. Overnight fasting of animals prior to blood sampling is recommended.
(i) Hematology. The recommended parameters are red blood cell count, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration, white blood cell count, differential leukocyte count, platelet count, and a measure of clotting potential, such as prothrombin time or activated partial thromboplastin time.
(ii) Clinical chemistry. (A) Parameters which are considered appropriate to all studies are electrolyte balance, carbohydrate metabolism, and liver and kidney function. The selection of specific tests will be influenced by observations on the mode of action of the substance and signs of clinical toxicity.
(B) The recommended clinical chemistry determinations are potassium, sodium, glucose, total cholesterol, urea nitrogen, creatinine, total protein, and albumin. More than two hepatic enzymes, (such as alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, sorbitol dehydrogenase, or gamma glutamyl transpeptidase) should also be measured. Measurements of addtional enzymes (of hepatic or other origin) and bile acids, may also be useful.
(iii) If a test chemical has an effect on the hematopoietic system, reticulocyte counts and bone marrow cytology may be indicated.
(iv) Other determinations that should be carried out if the test chemical is known or suspected of affecting related measures include calcium, phosphorus, fasting triglycerides, hormones, methemoglobin, and cholinesterases.
(v) Urinalyses. Urinalysis for rodents must be performed at the end of the first year of the study using timed urine collection. Urinalysis determinations include: appearance, volume, osmolality Start Printed Page 78806or specific gravity, pH, protein, glucose, and blood/blood cells.
(9) Ophthalmological examination. Examinations must be made on all animals using an ophthalmoscope or an equivalent device prior to the administration of the test substance and at termination of the study on 10 animals per sex in the high-dose and control groups. If changes in eyes are detected, all animals must be examined.
(10) Gross necropsy. (i) A complete gross examination must be performed on all animals, including those which died during the experiment or were sacrificed in a moribund condition.
(ii) At least, the liver, kidneys, adrenals, testes, epididymides, ovaries, uterus, spleen, brain, and heart should be trimmed and weighed wet, as soon as possible after dissection to avoid drying. The lungs should be weighed if the test substance is administered by the inhalation route. The organs should be weighed from interim sacrifice animals as well as from at least 10 animals per sex per group at terminal sacrifice.
(iii) The following organs and tissues, or representative samples thereof, must be preserved in a suitable medium for possible future histopathological examination:
(A) Digestive system—salivary glands, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, liver, pancreas, gallbladder (when present) .
(B) Nervous system—brain (multiple sections, including cerebrum, cerebellum and medulla/pons), pituitary, peripheral nerve (sciatic or tibial, preferably in close proximity to the muscle), spinal cord (three levels, cervical, mid-thoracic, and lumbar), eyes (retina, optic nerve).
(C) Glandular system—adrenals, parathyroid, thyroid.
(D) Respiratory system—trachea, lungs, pharynx, larynx, nose.
(E) Cardiovascular/Hematopoietic system—aorta, heart, bone marrow (and/or fresh aspirate), lymph nodes (preferably one lymph node covering the route of administration and another one distant from the route of administration to cover systemic effects), spleen.
(F) Urogenital system—kidneys, urinary bladder, prostate, testes, epididymides, seminal vesicle(s), uterus, ovaries, female mammary gland.
(G) Other—all gross lesions and masses, skin.
(iv) In inhalation studies, the entire respiratory tract, including nose, pharynx, larynx, and paranasal sinuses should be examined and preserved. In dermal studies, skin from treated and adjacent control skin sites should be examined and preserved.
(v) Inflation of lungs and urinary bladder with a fixative is the optimal method for preservation of these tissues. The proper inflation and fixation of the lungs in inhalation studies is essential for appropriate and valid histopathological examination.
(vi) Information from clinical pathology and other in-life data should be considered before microscopic examination, since these data may provide significant guidance to the pathologist.
(11) [Reserved]
(12) Histopathology. (i) The following histopathology must be performed:
(A) Full histopathology on the organs and tissues, listed in paragraph (e)(10)(iii) of this section of all animals in the control and high dose groups and of all animals that died or were sacrificed during the study.
(B) All gross lesions in all animals.
(C) Target organs in all animals.
(ii) If the results show substantial alteration of the animal's normal life span, the induction of effects that might affect a neoplastic response, or other effects that might compromise the significance of the data, the next lower levels should be examined fully as described in paragraph (e)(12)(i) of this section.
(iii) An attempt should be made to correlate gross observations with microscopic findings.
(iv) Tissues and organs designated for microscopic examination should be fixed in 10% buffered formalin or a recognized suitable fixative as soon as necropsy is performed and no less than 48 hours prior to trimming.
(f) Data and reporting—(1) Treatment of results. (i) Data must be summarized in tabular form, showing for each test group the number of animals at the start of the test, the number of animals showing lesions, the types of lesions and the percentage of animals displaying each type of lesion.
(ii) When applicable, all observed results, quantitative and qualitative, must be evaluated by an appropriate statistical method. Any generally accepted statistical methods may be used; the statistical methods including significance criteria should be selected during the design of the study.
(2) Evaluation of study results. (i) The findings of a combined chronic toxicity/carcinogenicity study should be evaluated in conjunction with the findings of previous studies and considered in terms of the toxic effects, the necropsy and histopathological findings. The evaluation must include the relationship between the dose of the test substance and the presence, incidence and severity of abnormalities (including behavioral and clinical abnormalities), gross lesions, identified target organs, body weight changes, effects on mortality and any other general or specific toxic effects.
(ii) In any study which demonstrates an absence of toxic effects, further investigation to establish absorption and bioavailablity of the test substance should be considered.
(iii) In order for a negative test to be acceptable, it should meet the following criteria—no more than 10% of any group is lost due to autolysis, cannibalism, or management problems, and survival in each group is no less than 50% at 15 months for mice and 18 months for rats. Survival should not fall below 25% at 18 months for mice and 24 months for rats.
(iv) The use of historical control data from an appropriate time period from the same testing laboratory (i.e, the incidence of tumors and other suspect lesions normally occurring under the same laboratory conditions and in the same strain of animals employed in the test) is helpful for assessing the significance of changes observed in the current study.
(3) Test report. (i) In addition to the reporting requirements specified under EPA Good Laboratory Practice Standards at 40 CFR part 792, subpart J, the following specific information must be reported:
(A) Test substance characterization should include:
(1) Chemical identification.
(2) Lot or batch number.
(3) Physical properties.
(4) Purity/impurities.
(5) Identification and composition of any vehicle used.
(B) Test system should contain data on:
(1) Species and strain of animals used and rationale for selection if other than that recommended.
(2) Age including body weight data and sex.
(3) Test environment including cage conditions, ambient temperature, humidity, and light/dark periods.
(4) Identification of animal diet.
(5) Acclimation period.
(C) Test procedure should include the following data:
(1) Method of randomization used.
(2) Full description of experimental design and procedure.
(3) Dose regimen including levels, methods, and volume.
(4) Test results. (i) Group animal data. Tabulation of toxic response data by species, strain, sex, and exposure level for: Start Printed Page 78807
(A) Number of animals exposed.
(B) Number of animals showing signs of toxicity.
(C) Number of animals dying.
(ii) Individual animal data. Data should be presented as summary (group mean) as well as for individual animals.
(A) Time of death during the study or whether animals survived to termination.
(B) Time of observation of each abnormal sign and its subsequent course.
(C) Body weight data.
(D) Feed and water consumption data, when collected.
(E) Achieved dose (milligrams/kilogram body weight) as a time-weighed average is the test substance is administered in the diet or drinking water.
(F) Results of ophthalmological examination, when performed.
(G) Results of hematological tests performed.
(H) Results of clinical chemistry tests performed.
(I) Results of urinalysis tests performed.
(J) Results of observations made.
(K) Necropsy findings including absolute/relative organ weight data.
(L) Detailed description of all histopathological findings.
(M) Statistical treatment of results where appropriate.
(N) Historical control data.
(iii) In addition, for inhalation studies the following should be reported:
(A) Test conditions. The following exposure conditions must be reported.
(1) Description of exposure apparatus including design, type, dimensions, source of air, system for generating particulates and aerosols, method of conditioning air, treatment of exhaust air and the method of housing the animals in a test chamber.
(2) The equipment for measuring temperature, humidity, and particulate aerosol concentrations and size should be described.
(B) Exposure data. These must be tabulated and presented with mean values and a measure of variability (e.g., standard deviation) and should include:
(1) Airflow rates through the inhalation equipment.
(2) Temperature and humidity of air.
(3) Actual (analytical or gravimetric) concentration in the breathing zone.
(4) Nominal concentration (total amount of test substance fed into the inhalation equipment divided by volume of air).
(5) Particle size distribution, and calculated MMAD and geometric standard deviation.
(6) Explanation as to why the desired chamber concentration and/or particle size could not be achieved (if applicable) and the efforts taken to comply with this aspect of the guidelines.
(g) Quality control. A system must be developed and maintained to assure and document adequate performance of laboratory equipment. The study must be conducted in compliance with 40 CFR Part 792—Good Laborary Practice Standards.
(h) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., NW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Benitz, K.F. Measurement of Chronic Toxicity. Methods of Toxicology. Ed. G.E. Paget. Blackwell, Oxford. pp. 82-131 (1970).
(2) Crofton K.M., Howard J.L., Moser V.C., Gill M.W., Leiter L.W., Tilson H.A., MacPhail, R.C. Interlaboratory Comparison of Motor Activity Experiments: Implication for Neurotoxicological Assessments. Neurotoxicol. Teratol. 13, 599-609. (1991)
(3) D'Aguanno, W. Drug Safety Evaluation—Pre-Clinical Considerations. Industrial Pharmacology: Neuroleptic. Vol. I, Ed. S. Fielding and H. Lal. Futura, Mt. Kisco, NY. pp. 317-332 (1974).
(4) Fitzhugh, O.G. Chronic Oral Toxicity, Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics. The Association of Food and Drug Officials of the United States. pp. 36-45 (1959, 3rd Printing 1975).
(5) Goldenthal, E.I. and D'Aguanno, W. Evaluation of Drugs, Appraisal of the Safety of Chemicals in Foods, Drugs, and Cosmetics. The Association of Food and Drug Officials of the United States. pp. 60-67 (1959, 3rd Printing 1975).
(6) Organization for Economic Cooperation and Development. Guidelines for Testing of Chemicals, Section 4-Health Effects, Part 453 Combined Chronic Toxicity/Carcinogenicity Studies, Paris. (1981).
(7) Page, N.P. Chronic Toxicity and Carcinogenicity Guidelines. Journal of Environmental Pathology and Toxicology 11:161-182 (1977).
(8) Page, N.P. Concepts of a Bioassay Program in Environmental Carcinogenesis, Advances in Modern Toxicology. Vol.3, Ed. Kraybill and Mehlman. Hemisphere, Washington, DC pp. 87-171 (1977)
(9) Sontag, J.M. et al. Guidelines for Carcinogen Bioassay in Small Rodents. NCI-CS-TR-1 (Bethesda: United States Cancer Institute, Division of Cancer Control and Prevention, Carcinogenesis Bioassay Program.
(10) Summary of the EPA Workshop on Carcinogenesis Bioassay via the Dermal Route. EPA Report 50/6-89-002; 50/6-89-003. Washington, DC.
(11) The Atlas Of Dermal Lesions, EPA Report 20T-004, U.S Environmental Protection Agency, Washington, DC.
TSCA in vitro mammalian chromosome aberration test.(a) Scope—(1) Applicability. This section is intended to meet testing requirements under section 4 of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Background. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides, and Toxic Substances (OPPTS) harmonized test guideline 870.5375 (August 1998, final guidelines). The source is available at the address in paragraph (i) of this section.
(b) Purpose. (1) The purpose of the in vitro chromosome aberration test is to identify agents that cause structural chromosome aberrations in cultured mammalian cells (see paragraphs (i)(1), (i)(2), and (i)(3) of this section). Structural aberrations may be of two types, chromosome or chromatid. With the majority of chemical mutagens, induced aberrations are of the chromatid type, but chromosome-type aberrations also occur. An increase in polyploidy may indicate that a chemical has the potential to induce numerical aberrations. However, this guideline is not designed to measure numerical aberrations and is not routinely used for that purpose. Chromosome mutations and related events are the cause of many human genetic diseases and there is substantial evidence that chromosome mutations and related events causing alterations in oncogenes and tumour-suppressor genes of somatic cells are involved in cancer induction in humans and experimental animals.
(2) The in vitro chromosome aberration test may employ cultures of established cell lines, cell strains or primary cell cultures. The cells used are selected on the basis of growth ability in culture, stability of the karyotype, chromosome number, chromosome diversity, and spontaneous frequency of chromosome aberrations.
(c) Definitions. The definitions in section 3 of TSCA and in 40 CFR Part 792—Good Laboratory Practice Start Printed Page 78808Standards apply to this test guideline. The following definitions also apply to this test guideline.
Chromatid-type aberration is structural chromosome damage expressed as breakage of single chromatids or breakage and reunion between chromatids.
Chromosome-type aberration is structural chromosome damage expressed as breakage, or breakage and reunion, of both chromatids at an identical site.
Endoreduplication is a process in which after an S period of DNA replication, the nucleus does not go into mitosis but starts another S period. The result is chromosomes with 4, 8, 16,...chromatids.
Gap is an achromatic lesion smaller than the width of one chromatid, and with minimum misalignment of the chromatid(s).
Mitotic index is the ratio of cells in metaphase divided by the total number of cells observed in a population of cells; an indication of the degree of proliferation of that population.
Numerical aberration is a change in the number of chromosomes from the normal number characteristic of the cells utilized.
Polyploidy is a multiple of the haploid chromosome number (n) other than the diploid number (i.e., 3n, 4n, and so on).
Structural aberration is a change in chromosome structure detectable by microscopic examination of the metaphase stage of cell division, observed as deletions and fragments, intrachanges, and interchanges.
(d) Initial considerations. (1) Tests conducted in vitro generally require the use of an exogenous source of metabolic activation. This metabolic activation system cannot mimic entirely the mammalian in vivo conditions. Care should be taken to avoid conditions which would lead to positive results which do not reflect intrinsic mutagenicity and may arise from changes in pH, osmolality, or high levels of cytotoxicity (the test techniques described in the references under paragraphs (i)(4) and (i)(5) of this section may be used).
(2) This test is used to screen for possible mammalian mutagens and carcinogens. Many compounds that are positive in this test are mammalian carcinogens; however, there is not a perfect correlation between this test and carcinogenicity. Correlation is dependent on chemical class and there is increasing evidence that there are carcinogens that are not detected by this test because they appear to act through mechanisms other than direct DNA damage.
(e) Principle of the test method. Cell cultures are exposed to the test substance both with and without metabolic activation. At predetermined intervals after exposure of cell cultures to the test substance, they are treated with a metaphase-arresting substance (e.g., Colcemid® or colchicine), harvested, stained, and metaphase cells are analysed microscopically for the presence of chromosome aberrations.
(f) Description of the method—(1) Preparations—(i) Cells. A variety of cell lines, strains, or primary cell cultures, including human cells, may be used (e.g., Chinese hamster fibroblasts, human, or other mammalian peripheral blood lymphocytes).
(ii) Media and culture conditions. Appropriate culture media, and incubation conditions (culture vessels, CO2 concentration, temperature and humidity) must be used in maintaining cultures. Established cell lines and strains must be checked routinely for stability in the modal chromosome number and the absence of Mycoplasma contamination and should not be used if contaminated. The normal cell-cycle time for the cells and culture conditions used should be known.
(iii) Preparation of cultures—(A) Established cell lines and strains. Cells are propagated from stock cultures, seeded in culture medium at a density such that the cultures will not reach confluency before the time of harvest, and incubated at 37 °C.
(B) Lymphocytes. Whole blood treated with an anti-coagulant (e.g., heparin) or separated lymphocytes obtained from healthy subjects are added to culture medium containing a mitogen (e.g., phytohemagglutinin) and incubated at 37 °C.
(iv) Metabolic activation. Cells must be exposed to the test substance both in the presence and absence of an appropriate metabolic activation system. The most commonly used system is a co-factor-supplemented post-mitochondrial fraction (S9) prepared from the livers of rodents treated with enzyme-inducing agents such as Aroclor 1254 (the test techniques described in the references under paragraphs (i)(6), (i)(7), (8)(i), and (i)(9) of this section may be used), or a mixture of phenobarbitone and β-naphthoflavone (the test techniques described in the references under paragraphs (i)(10), (i)(11), and (i)(12) of this section may be used). The post-mitochondrial fraction is usually used at concentrations in the range from 1-10% v/v in the final test medium. The condition of a metabolic activation system may depend upon the class of chemical being tested. In some cases, it may be appropriate to utilize more than one concentration of post-mitochondrial fraction. A number of developments, including the construction of genetically engineered cell lines expressing specific activating enzymes, may provide the potential for endogenous activation. The choice of the cell lines used should be scientifically justified (e.g., by the relevance of the cytochrome P450 isoenzyme for the metabolism of the test substance).
(v) Test substance/preparation. Solid test substances should be dissolved or suspended in appropriate solvents or vehicles and diluted, if appropriate, prior to treatment of the cells. Liquid test substances may be added directly to the test systems and/or diluted prior to treatment. Fresh preparations of the test substance should be employed unless stability data demonstrate the acceptability of storage.
(2) Test conditions—(i) Solvent/vehicle. The solvent/vehicle should not be suspected of chemical reaction with the test substance and must be compatible with the survival of the cells and the S9 activity. If other than well-known solvent/vehicles are used, their inclusion should be supported by data indicating their compatibility. It is recommended that wherever possible, the use of an aqueous solvent/vehicle be considered first. When testing water-unstable substances, the organic solvents used should be free of water. Water can be removed by adding a molecular sieve.
(ii) Exposure concentrations. (A) Among the criteria to be considered when determining the highest concentration are cytotoxicity, solubility in the test system, and changes in pH or osmolality.
(B) Cytotoxicity should be determined with and without metabolic activation in the main experiment using an appropriate indication of cell integrity and growth, such as degree of confluency, viable cell counts, or mitotic index. It may be useful to determine cytotoxicity and solubility in a preliminary experiment.
(C) At least three analyzable concentrations should be used. Where cytotoxicity occurs, these concentrations should cover a range from the maximum to little or no toxicity; this will usually mean that the concentrations should be separated by no more than a factor between 2 and t10. At the time of harvesting, the highest concentration should show a significant reduction in degree of confluency, cell count or mitotic index, (all greater than 50%). The mitotic Start Printed Page 78809index is only an indirect measure of cytotoxic/cytostatic effects and depends on the time after treatment. However, the mitotic index is acceptable for suspension cultures in which other toxicity measurements may be cumbersome and impractical. Information on cell-cycle kinetics, such as average generation time (AGT), could be used as supplementary information. AGT, however, is an overall average that does not always reveal the existence of delayed subpopulations, and even slight increases in average generation time can be associated with very substantial delay in the time of optimal yield of aberrations. For relatively non-cytotoxic compounds the maximum concentration should be 5 mg/ml, 5mg/ml, or 0.01M, whichever is the lowest.
(D) For relatively insoluble substances that are not toxic at concentrations lower than the insoluble concentration, the highest dose used should be a concentration above the limit of solubility in the final culture medium at the end of the treatment period. In some cases (e.g., when toxicity occurs only at higher than the lowest insoluble concentration) it is advisable to test at more than one concentration with visible precipitation. It may be useful to assess solubility at the beginning and the end of the treatment, as solubility can change during the course of exposure in the test system due to presence of cells, S9, serum etc. Insolubility can be detected by using the unaided eye. The precipitate should not interfere with the scoring.
(iii) Controls. (A) Concurrent positive and negative (solvent or vehicle) controls both with and without metabolic activation must be included in each experiment. When metabolic activation is used, the positive control chemical must be the one that requires activation to give a mutagenic response.
(B) Positive controls must employ a known clastogen at exposure levels expected to give a reproducible and detectable increase over background which demonstrates the sensitivity of the test system. Positive control concentrations should be chosen so that the effects are clear but do not immediately reveal the identity of the coded slides to the reader. Examples of positive-control substances include:
Metabolic activation condition Chemical CAS number Absence of exogenous metabolic activation Methyl methanesulfonate [66-27-3] Ethyl methanesulfonate [62-50-0] Ethylnitrosourea [759-73-9] Mitomycin C [50-07-7] 4-Nitroquinoline-N-Oxide [56-57-5] Presence of exogenous metabolic activation Benzo(a)pyrene [50-32-8] Cyclophosphamide (monohydrate) [50-18-0] ([6055-19-2]) (C) Other appropriate positive control substances may be used. The use of chemical class-related positive-control chemicals may be considered, when available.
(D) Negative controls, consisting of solvent or vehicle alone in the treatment medium, and treated in the same way as the treatment cultures, must be included for every harvest time. In addition, untreated controls should also be used unless there are historical-control data demonstrating that no deleterious or mutagenic effects are induced by the chosen solvent.
(g) Procedure—(1) Treatment with test substance. (i) Proliferating cells are treated with the test substance in the presence and absence of a metabolic-activation system. Treatment of lymphocytes should commence at about 48 hours after mitogenic stimulation.
(ii) Duplicate cultures must be used at each concentration, and are strongly recommended for negative/solvent control cultures. Where minimal variation between duplicate cultures can be demonstrated (the test techniques described in the references under paragraphs (i)(13) and (i)(14) of this section may be used), from historical data, it may be acceptable for single cultures to be used at each concentration.
(iii) Gaseous or volatile substances should be tested by appropriate methods, such as in sealed culture vessels (the test techniques described in the references under paragraphs (i)(15) and (i)(16) of this section may be used).
(2) Culture harvest time. In the first experiment, cells should be exposed to the test substance both with and without metabolic activation for 3-6 hours, and sampled at a time equivalent to about 1.5 normal cell-cycle length after the beginning of treatment (the test techniques described in the references under paragraph (i)(12) of this section may be used). If this protocol gives negative results both with and without activation, an additional experiment without activation should be done, with continuous treatment until sampling at a time equivalent to about 1.5 normal cell-cycle lengths. Certain chemicals may be more readily detected by treatment/sampling times longer than 1.5 cycle lengths. Negative results with metabolic activation need to be confirmed on a case-by-case basis. In those cases where confirmation of negative results is not considered necessary, justification should be provided.
(3) Chromosome preparation. Cell cultures must be treated with Colcemid® or colchicine usually for 1 to 3 hours prior to harvesting. Each cell culture must be harvested and processed separately for the preparation of chromosomes. Chromosome preparation involves hypotonic treatment of the cells, fixation and staining.
(4) Analysis. (i) All slides, including those of positive and negative controls, must be independently coded before microscopic analysis. Since fixation procedures often result in the breakage of a proportion of metaphase cells with loss of chromosomes, the cells scored must therefore contain a number of centromeres equal to the modal number ± 2 for all cell types. At least 200 well-spread metaphases should be scored per concentration and control equally divided amongst the duplicates, if applicable. This number can be reduced when high numbers of aberrations are observed.
(ii) Though the purpose of the test is to detect structural chromosome aberrations, it is important to record polyploidy and endoreduplication when these events are seen.
(h) Data and reporting—(1) Treatment of results. (i) The experimental unit is the cell, and therefore the percentage of cells with structural chromosome aberration(s) should be evaluated. Different types of structural Start Printed Page 78810chromosome aberrations must be listed with their numbers and frequencies for experimental and control cultures. Gaps are recorded separately and reported but generally not included in the total aberration frequency.
(ii) Concurrent measures of cytotoxicity for all treated and negative control cultures in the main aberration experiment(s) should also be recorded.
(iii) Individual culture data should be provided. Additionally, all data should be summarized in tabular form.
(iv) There is no requirement for verification of a clear positive response. Equivocal results should be clarified by further testing preferably using modification of experimental conditions. The need to confirm negative results has been discussed in paragraph (g)(2) of this section. Modification of study parameters to extend the range of conditions assessed should be considered in follow-up experiments. Study parameters that might be modified include the concentration spacing and the metabolic activation conditions.
(2) Evaluation and interpretation of results. (i) There are several criteria for determining a positive result, such as a concentration-related increase or a reproducible increase in the number of cells with chromosome aberrations. Biological relevance of the results should be considered first. Statistical methods may be used as an aid in evaluating the test results (see paragraphs (i)(3) and (i)(13) of this section). Statistical significance should not be the only determining factor for a positive response.
(ii) An increase in the number of polyploid cells may indicate that the test substance has the potential to inhibit mitotic processes and to induce numerical chromosome aberrations. An increase in the number of cells with endoreduplicated chromosomes may indicate that the test substance has the potential to inhibit cell-cycle progression (the test techniques described in the references under paragraphs (i)(17) and (i)(18) of this section may be used).
(iii) A test substance for which the results do not meet the criteria in paragraphs (h)(2)(i) and (h)(2)(ii) of this section is considered nonmutagenic in this system.
(iv) Although most experiments will give clearly positive or negative results, in rare cases the data set will preclude making a definite judgement about the activity of the test substance. Results may remain equivocal or questionable regardless of the number of times the experiment is repeated.
(v) Positive results from the in vitro chromosome aberration test indicate that the test substance induces structural chromosome aberrations in cultured mammalian somatic cells. Negative results indicate that, under the test conditions, the test substance does not induce chromosome aberrations in cultured mammalian somatic cells.
(3) Test report. The test report must include the following information.
(i) Test substance.
(A) Identification data and CAS no., if known.
(B) Physical nature and purity.
(C) Physicochemical properties relevant to the conduct of the study.
(D) Stability of the test substance, if known.
(ii) Solvent/vehicle.
(A) Justification for choice of solvent/vehicle.
(B) Solubility and stability of the test substance in solvent/vehicle, if known.
(iii) Cells.
(A) Type and source of cells.
(B) Karyotype features and suitability of the cell type used.
(C) Absence of Mycoplasma, if applicable.
(D) Information on cell-cycle length.
(E) Sex of blood donors, whole blood or separated lymphocytes, mitogen used.
(F) Number of passages, if applicable.
(G) Methods for maintenance of cell cultures if applicable.
(H) Modal number of chromosomes.
(iv) Test conditions.
(A) Identity of metaphase arresting substance, its concentration and duration of cell exposure.
(B) Rationale for selection of concentrations and number of cultures including, e.g., cytotoxicity data and solubility limitations, if available.
(C) Composition of media, CO2 concentration if applicable.
(D) Concentration of test substance.
(E) Volume of vehicle and test substance added.
(F) Incubation temperature.
(G) Incubation time.
(H) Duration of treatment.
(I) Cell density at seeding, if appropriate.
(J) Type and composition of metabolic activation system, including acceptability criteria.
(K) Positive and negative controls.
(L) Methods of slide preparation.
(M) Criteria for scoring aberrations.
(N) Number of metaphases analyzed.
(O) Methods for the measurements of toxicity.
(P) Criteria for considering studies as positive, negative or equivocal.
(v) Results.
(A) Signs of toxicity, e.g., degree of confluency, cell-cycle data, cell counts, mitotic index.
(B) Signs of precipitation.
(C) Data on pH and osmolality of the treatment medium, if determined.
(D) Definition for aberrations, including gaps.
(E) Number of cells with chromosome aberrations and type of chromosome aberrations given separately for each treated and control culture.
(F) Changes in ploidy if seen.
(G) Dose-response relationship, where possible.
(H) Statistical analyses, if any.
(I) Concurrent negative (solvent/vehicle) and positive control data.
(J) Historical negative (solvent/vehicle) and positive control data, with ranges, means and standard deviations.
(vi) Discussion of the results.
(vii) Conclusion.
(i) References. For additional background information on this test guideline, the following references should be consulte. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., SW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Evans, H.J. Cytological Methods for Detecting Chemical Mutagens. Chemical Mutagens, Principles and Methods for their Detection, Vol. 4, Hollaender, A. Ed. Plenum Press, New York and London, pp. 1-29 (1976).
(2) Ishidate, M. Jr. and Sofuni, T. The In Vitro Chromosomal Aberration Test Using Chinese Hamster Lung (CHL) Fibroblast Cells in Culture. Progress in Mutation Research, Vol. 5, Ashby, J. et al., Eds. Elsevier Science Publishers, Amsterdam-New York-Oxford, pp. 427-432 (1985).
(3) Galloway, S.M. et al. Chromosome aberration and sister chromatid exchanges in Chinese hamster ovary cells: Evaluation of 108 chemicals. Environmental and Molecular Mutagenesis 10 (suppl. 10), 1-175 (1987).
(4) Scott, D. et al.. Genotoxicity under Extreme Culture Conditions. A report from ICPEMC Task Group 9. Mutation Research 257, 147-204 (1991).
(5) Morita, T. et al. Clastogenicity of Low pH toVarious Cultured Mammalian Cells. Mutation Research 268, 297-305 (1992).
(6) Ames, B.N., McCann, J. and Yamasaki, E. Methods for Detecting Carcinogens and Mutagens with the Salmonella/Mammalian Microsome Mutagenicity Test. Mutation Research 31, 347-364 (1975).
(7) Maron, D.M. and Ames, B.N. Revised Methods for the Salmonella Mutagenicity Test. Mutation Research 113, 173-215 (1983). Start Printed Page 78811
(8) Natarajan, A.T. et al. Cytogenetic Effects of Mutagens/Carcinogens after Activation in a Microsomal System In Vitro, I. Induction of Chromosome Aberrations and Sister Chromatid Exchanges by Diethylnitrosamine (DEN) and Dimethylnitrosamine (DMN) in CHO Cells in the Presence of Rat-Liver Microsomes. Mutation Research 37, 83-90 (1976).
(9) Matsuoka, A., Hayashi, M. and Ishidate, M., Jr. Chromosomal Aberration Tests on 29 Chemicals Combined with S9 Mix In Vitro. Mutation Research 66, 277-290 (1979).
(10) Elliot, B.M. et al. Report of UK Environmental Mutagen Society Working Party. Alternatives to Aroclor 1254-induced S9 in In Vitro Genotoxicity Assays. Mutagenesis 7, 175-177 (1992).
(11) Matsushima, T. et al. A Safe Substitute for Polychlorinated Biphenyls as an Inducer of Metabolic Activation Systems. de Serres, F.J., Fouts, J.R., Bend, J.R. and Philpot, R.M. Eds. In Vitro Metabolic Activation in Mutagenesis Testing, Elsevier, North-Holland, pp. 85-88 (1976).
(12) Galloway, S.M. et al. Report from Working Group on In Vitro Tests for Chromosomal Aberrations. Mutation Research 312, 241-261 (1994).
(13) Richardson, C. et al. Analysis of Data from In Vitro Cytogenetic Assays. Statistical Evaluation of Mutagenicity Test Data. Kirkland, D.J., Ed. Cambridge University Press, Cambridge, pp. 141-154 (1989).
(14) Soper, K.A. and Galloway S.M. Replicate Flasks are not Necessary for In Vitro Chromosome Aberration Assays in CHO Cells. Mutation Research 312, 139-149 (1994).
(15) Krahn, D.F., Barsky, F.C. and McCooey, K.T. CHO/HGPRT Mutation Assay: Evaluation of Gases and Volatile Liquids. Tice, R.R., Costa, D.L., Schaich, K.M. Eds. Genotoxic Effects of Airborne Agents. New York, Plenum, pp. 91-103 (1982).
(16) Zamora, P.O. et al. Evaluation of an Exposure System Using Cells Grown on Collagen Gels for Detecting Highly Volatile Mutagens in the CHO/HGPRT Mutation Assay. Environmental Mutagenesis 5, 795-801 (1983).
(17) Locke-Huhle, C. Endoreduplication in Chinese hamster cells during alpha-radiation induced G2 arrest. Mutation Research 119, 403-413 (1983).
(18) Huang, Y., Change, C. and Trosko, J.E. Aphidicolin—induced endoreduplication in Chinese hamster cells. Cancer Research 43, 1362-1364 (1983).
TSCA developmental neurotoxicity.(a) Scope—(1) Applicability. This section is intended to meet the testing requirements under section 4 of the Toxic Substances Control Act (TSCA).
(2) Source. The source material used in developing this TSCA test guideline is the OPPTS harmonized test guideline 870.6300 (August 1998).
(b) Purpose. In the assessment and evaluation of the toxic characteristics of a chemical substance or mixture (test substance), determination of the potential for developmental neurotoxicity is important. This study is designed to develop data on the potential functional and morphological hazards to the nervous system which may arise in the offspring from exposure of the mother during pregnancy and lactation.
(c) Principle of the test method. The test substance is administered to several groups of pregnant animals during gestation and early lactation, one dose level being used per group. Offspring are randomly selected from within litters for neurotoxicity evaluation. The evaluation includes observations to detect gross neurologic and behavioral abnormalities, determination of motor activity, response to auditory startle, assessment of learning, neuropathological evaluation, and brain weights. This protocol may be used as a separate study, as a followup to a standard developmental toxicity and/or adult neurotoxicity study, or as part of a two-generation reproduction study, with assessment of the offspring conducted on the second (F2) generation.
(d) Test procedure—(1) Animal selection—(i) Species and strain. Testing must be performed in the rat. Because of its differences in timing of developmental events compared to strains that are more commonly tested in other developmental and reproductive toxicity studies, it is preferred that the Fischer 344 strain not be used. If a sponsor wishes to use the Fischer 344 rat or a mammalian species other than the rat, ample justification/reasoning for this selection must be provided.
(ii) Age. Young adult (nulliparous females) animals must be used.
(iii) Sex. Pregnant female animals must be used at each dose level.
(iv) Number of animals. (A) The objective is for a sufficient number of pregnant rats to be exposed to the test substance to ensure that an adequate number of offspring are produced for neurotoxicity evaluation. At least 20 litters are recommended at each dose level.
(B) On postnatal day 4, the size of each litter should be adjusted by eliminating extra pups by random selection to yield, as nearly as possible, four male and four females per litter. Whenever the number of pups of either sex prevents having four of each sex per litter, partial adjustment (for example, five males and three females) is permitted. Testing is not appropriate for litters of less than seven pups. Elimination of runts only is not appropriate. Individual pups should be identified uniquely after standardization of litters. A method that may be used for identification can be found under paragraph (f)(1) of this section.
(v) Assignment of animals for behavioral tests, brain weights, and neuropathological evaluations. After standardization of litters, one male or one female from each litter (total of 10 males and 10 females per dose group) must be randomly assigned to one of the following tests: Motor activity, auditory startle, and learning and memory, in weanling and adult animals. On postnatal day 11, either 1 male or 1 female pup from each litter (total of 10 males and 10 females per dose group) must be sacrificed. Brain weights must be measured in all of these pups and, of these pups, six per sex per dose must be selected for neuropathological evaluation. At the termination of the study, either 1 male or 1 female from each litter (total of 10 males and 10 females per dose group) must be sacrificed and brain weights must be measured. An additional group of six animals per sex per dose group (one male or one female per litter) must be sacrificed at the termination of the study for neuropathological evaluation.
(2) Control group. A concurrent control group is required. This group must be a sham-treated group or, if a vehicle is used in administering the test substance, a vehicle control group. The vehicle must neither be developmentally toxic nor have effects on reproduction. Animals in the control group must be handled in an identical manner to test group animals.
(3) Dose levels and dose selection. (i) At least three dose levels of the test substance plus a control group (vehicle control, if a vehicle is used) must be used.
(ii) If the test substance has been shown to be developmentally toxic either in a standard developmental toxicity study or in a pilot study, the highest dose level must be the maximum dose which will not induce in utero or neonatal death or malformations sufficient to preclude a meaningful evaluation of neurotoxicity. Start Printed Page 78812
(iii) If a standard developmental toxicity study has not been conducted, the highest dose level, unless limited by the physicochemical nature or biological properties of the substance, must induce some overt maternal toxicity, but must not result in a reduction in weight gain exceeding 20 percent during gestation and lactation.
(iv) The lowest dose should not produce any grossly observable evidence of either maternal or developmental neurotoxicity.
(v) The intermediate doses must be equally spaced between the highest and lowest doses used.
(4) Dosing period. Day 0 of gestation is the day on which a vaginal plug and/or sperm are observed. The dosing period must cover the period from day 6 of gestation through day 10 postnatally. Dosing should not occur on the day of parturition in those animals who have not completely delivered their offspring.
(5) Administration of the test substance. The test substance or vehicle must be administered orally. Other routes of administration may be acceptable, on a case-by-case basis, with ample justification/reasoning for this selection. The test substance or vehicle must be administered based on the most recent weight determination.
(6) Observation of dams. (i) A gross examination of the dams must be made at least once each day before daily treatment.
(ii) Ten dams per group must be observed outside the home cage at least twice during the gestational dosing period (days 6-21) and twice during the lactational dosing period (days 1-10) for signs of toxicity. The animals must be observed by trained technicians who are unaware of the animals' treatment, using standardized procedures to maximize interobserver reliability. Where possible, it is advisable that the same observer be used to evaluate the animals in a given study. If this is not possible, some demonstration of interobserver reliability is required.
(iii) During the treatment and observation periods under paragraph (d)(6)(ii) of this section, observations must include:
(A) Assessment of signs of autonomic function, including but not limited to:
(1) Ranking of the degree of lacrimation and salivation, with a range of severity scores from none to severe.
(2) Presence or absence of piloerection and exophthalmus.
(3) Ranking or count of urination and defecation, including polyuria and diarrhea.
(4) Pupillary function such as constriction of the pupil in response to light or a measure of pupil size.
(5) Degree of palpebral closure, e.g., ptosis.
(B) Description, incidence, and severity of any convulsions, tremors, or abnormal movements.
(C) Description and incidence of posture and gait abnormalities.
(D) Description and incidence of any unusual or abnormal behaviors, excessive or repetitive actions (stereotypies), emaciation, dehydration, hypotonia or hypertonia, altered fur appearance, red or crusty deposits around the eyes, nose, or mouth, and any other observations that may facilitate interpretation of the data.
(iv) Signs of toxicity must be recorded as they are observed, including the time of onset, degree, and duration.
(v) Animals must be weighed at least weekly and on the day of delivery and postnatal days 11 and 21 (weaning) and such weights must be recorded.
(vi) The day of delivery of litters must be recorded and considered as postnatal day 0.
(7) Study conduct—(i) Observation of offspring. (A) All offspring must be examined cage-side at least daily for gross signs of mortality or morbidity.
(B) A total of 10 male offspring and 10 female offspring per dose group must be examined outside the cage for signs of toxicity on days 4, 11, 21, 35, 45, and 60. The offspring must be observed by trained technicians, who are unaware of the treatment being used, using standardized procedures to maximize interobserver reliability. Where possible, it is advisable that the same observer be used to evaluate the animals in a given study. If this is not possible, some demonstration of interobserver reliability is required. At a minimum, the end points outlined in paragraph (d)(6)(iii) of this section must be monitored as appropriate for the developmental stage being observed.
(C) Any gross signs of toxicity in the offspring must be recorded as they are observed, including the time of onset, degree, and duration.
(ii) Developmental landmarks. Live pups must be counted and each pup within a litter must be weighed individually at birth or soon thereafter, and on postnatal days 4, 11, 17, and 21 and at least once every 2 weeks thereafter. The age of vaginal opening and preputial separation must be determined. General procedures for these determinations may be found in paragraphs (f)(1) and (f)(11) of this section.
(iii) Motor activity. Motor activity must be monitored specifically on postnatal days 13, 17, 21, and 60 (+2 days). Motor activity must be monitored by an automated activity recording apparatus. The device must be capable of detecting both increases and decreases in activity, (i.e., baseline activity as measured by the device must not be so low as to preclude detection of decreases nor so high as to preclude detection of increases in activity). Each device must be tested by standard procedures to ensure, to the extent possible, reliability of operation across devices and across days for any one device. In addition, treatment groups must be balanced across devices. Each animal must be tested individually. The test session must be long enough for motor activity to approach asymptotic levels by the last 20 percent of the session for nontreated control animals. All sessions must have the same duration. Treatment groups must be counter-balanced across test times. Activity counts must be collected in equal time periods of no greater than 10 minutes duration. Efforts must be made to ensure that variations in the test conditions are minimal and are not systematically related to treatment. Among the variables that can affect motor activity are sound level, size and shape of the test cage, temperature, relative humidity, light conditions, odors, use of home cage or novel test cage, and environmental distractions. Additional information on the conduct of a motor activity study may be obtained in § 799.9620.
(iv) Auditory startle test. An auditory startle habituation test should be performed on the offspring around the time of weaning and around day 60. Day of testing should be counterbalanced across treated and control groups. Details on the conduct of this testing may be obtained under paragraph (f)(1) of this section. In performing the auditory startle task, the mean response amplitude on each block of 10 trials (5 blocks of 10 trials per session on each day of testing) must be made. While use of prepulse inhibition is not a requirement, it is highly recommended. Details on the conduct of this test may be obtained in paragraph (f)(10) of this section.
(v) Learning and memory tests. A test of associative learning and memory should be conducted around the time of weaning and around day 60. Day of testing should be counterbalanced across treated and control groups. The same or separate tests may be used at these two stages of development. Some flexibility is allowed in the choice of tests for learning and memory in weanling and adult rats. However, the tests must be designed to fulfill two Start Printed Page 78813criteria. First, learning must be assessed either as a change across several repeated learning trials or sessions, or, in tests involving a single trial, with reference to a condition that controls for nonassociative effects of the training experience. Second, the tests must include some measure of memory (short-term or long-term) in addition to original learning (acquisition). If the tests of learning and memory reveal an effect of the test compound, it may be in the best interest of the sponsor to conduct additional tests to rule out alternative interpretations based on alterations in sensory, motivational, and/or motor capacities. In addition to the above two criteria, it is recommended that the test of learning and memory be chosen on the basis of its demonstrated sensitivity to the class of compound under investigation, if such information is available in the literature. In the absence of such information, examples of tests that could be made to meet the above criteria include: Delayed-matching-to-position, as described for the adult rat (see paragraph (f)(3) of this section) and for the infant rat (see paragraph (f)(9) of this section); olfactory conditioning, as described in paragraph (f)(13) of this section; and acquisition and retention of schedule-controlled behavior (see paragraphs (f)(4) and (f)(5) of this section). Additional tests for weanling rats are described under paragraphs (f)(20) and (f)(12) of this section, and for adult rats under paragraph (f)(16) of this section.
(vi) Neuropathology. Neuropathological evaluation must be conducted on animals on postnatal day 11 and at the termination of the study. At 11 days of age, one male or female pup must be removed from each litter such that equal numbers of male and female offspring are removed from all litters combined. Of these, six male and six female pups per dose group will be sacrificed for neuropathological analysis. The pups will be sacrificed by exposure to carbon dioxide and immediately thereafter the brains should be removed, weighed, and immersion-fixed in an appropriate aldehyde fixative. The remaining animals will be sacrificed in a similar manner and immediately thereafter their brains removed and weighed. At the termination of the study, one male or one female from each litter will be sacrificed by exposure to carbon dioxide and immediately thereafter the brain must be removed and weighed. In addition, six animals per sex per dose group (one male or female per litter) must be sacrificed at the termination of the study for neuropathological evaluation. Neuropathological analysis of animals sacrificed at the termination of the study must be performed in accordance with § 799.9620. Neuropathological evaluation of animals sacrificed on postnatal day 11 and at termination of the study must include a qualitative analysis and semiquantitative analysis as well as simple morphometrics.
(A) Fixation and processing of tissue samples for postnatal day 11 animals. Immediately following removal, the brain must be weighed and immersion fixed in an appropriate aldehyde fixative. The brains must be postfixed and processed according to standardized published histological protocols such as those discussed in references listed under paragraphs (f)(6), (f)(14), (f)(17), and (f)(21) of this section. Paraffin embedding is acceptable but plastic embedding is preferred and recommended. Tissue blocks and slides must be appropriately identified when stored. Histological sections must be stained for hematoxylin and eosin, or a similar stain according to standard published protocols such as those discussed in references listed under paragraphs (f)(2), (f)(18), and (f)(23) of this section. For animals sacrificed at the termination of the study, methods for fixation and processing of tissue samples are provided in § 799.9620(e)(7)(iv)(A).
(B) Qualitative analysis. The purposes of the qualitative examination are threefold—to identify regions within the nervous system exhibiting evidence of neuropathological alterations, to identify types of neuropathological alterations resulting from exposure to the test substance, and to determine the range of severity of the neuropathological alterations. Representative histological sections from the tissue samples should be examined microscopically by an appropriately trained pathologist for evidence of neuropathological alterations. The following stepwise procedure is recommended for the qualitative analysis. First, sections from the high dose group are compared with those of the control group. If no evidence of neuropathological alterations is found in animals of the high dose group, no further analysis is required. If evidence of neuropathological alterations are found in the high dose group, then animals from the intermediate and low dose group are examined. Subject to professional judgment and the kind of neuropathological alterations observed, it is recommended that additional methods such as Bodian's or Bielchowsky's silver methods and/or immunohistochemistry for glial fibrillary acid protein be used in conjunction with more standard stains to determine the lowest dose level at which neuropathological alterations are observed. Evaluations of postnatal day 11 pups is described in paragraphs (d)(7)(vi)(B)(1) and (d)(7)(vi)(B)(2) of this section. For animals sacrificed at the termination of the study, the regions to be examined and the types of alterations that must be assessed are identified in § 799.9620(e)(7)(iv)(B).
(1) Regions to be examined. The brains should be examined for any evidence of treatment-related neuropathological alterations and adequate samples should be taken from all major brain regions (e.g., olfactory bulbs, cerebral cortex, hippocampus, basal ganglia, thalamus, hypothalamus, midbrain (tectum, tegmentum, and cerebral peduncles), brainstem and cerebellum) to ensure a thorough examination.
(2) Types of alterations. Guidance for neuropathological examination for indications of developmental insult to the brain can be found in paragraphs (f)(8) and (f)(22) of this section. In addition to more typical kinds of cellular alterations (e.g., neuronal vacuolation, degeneration, necrosis) and tissue changes (e.g., astrocytic proliferation, leukocytic infiltration, and cystic formation) particular emphasis should be paid to structural changes indicative of developmental insult including but not restricted to:
(i) Gross changes in the size or shape of brain regions such as alterations in the size of the cerebral hemispheres or the normal pattern of foliation of the cerebellum.
(ii) The death of neuronal precursors, abnormal proliferation, or abnormal migration, as indicated by pyknotic cells or ectopic neurons, or gross alterations in regions with active proliferative and migratory zones, alterations in transient developmental structures (e.g., the external germinal zone of the cerebellum, see paragraph (f)(15) of this section).
(iii) Abnormal differentiation, while more apparent with special stains, may also be indicated by shrunken and malformed cell bodies.
(iv) Evidence of hydrocephalus, in particular enlargement of the ventricles, stenosis of the cerebral aqueduct and general thinning of the cerebral hemispheres.
(C) Subjective diagnosis. If any evidence of neuropathological alterations is found in the qualitative examination, then a subjective diagnosis will be performed for the purpose of Start Printed Page 78814evaluating dose-response relationships. All regions of the brain exhibiting any evidence of neuropathological changes must be included in this analysis. Sections of each region from all dose groups will be coded as to treatment and examined in randomized order. The frequency of each type and the severity of each lesion will be recorded. After all sections from all dose groups including all regions have been rated, the code will be broken and statistical analyses performed to evaluate dose-response relationships. For each type of dose related lesion observed, examples of different ranges of severity must be described. The examples will serve to illustrate a rating scale, such as 1+, 2+, and 3+ for the degree of severity ranging from very slight to very extensive.
(D) Simple morphometric analysis. Since the disruption of developmental processes is sometimes more clearly reflected in the rate or extent of growth of particular brain regions, some form of morphometric analysis must be performed on postnatal day 11 and at the termination of the study to assess the structural development of the brain. At a minimum, this would consist of a reliable estimate of the thickness of major layers at representative locations within the neocortex, hippocampus, and cerebellum. For guidance on such measurements see Rodier and Gramann under paragraph (f)(19) of this section.
(e) Data collection, reporting, and evaluation. The following specific information must be reported:
(1) Description of test system and test methods. A description of the general design of the experiment should be provided. This must include:
(i) A detailed description of the procedures used to standardize observations and procedures as well as operational definitions for scoring observations.
(ii) Positive control data from the laboratory performing the test that demonstrate the sensitivity of the procedures being used. These data do not have to be from studies using prenatal exposures. However, the laboratory must demonstrate competence in evaluation of effects in neonatal animals perinatally exposed to chemicals and establish test norms for the appropriate age group.
(iii) Procedures for calibrating and ensuring the equivalence of devices and the balancing of treatment groups in testing procedures.
(iv) A short justification explaining any decisions involving professional judgement.
(2) Results. The following information must be arranged by each treatment and control group:
(i) In tabular form, data for each animal must be provided showing:
(A) Its identification number and the litter from which it came.
(B) Its body weight and score on each developmental landmark at each observation time.
(C) Total session activity counts and intrasession subtotals on each day measured.
(D) Auditory startle response amplitude per session and intrasession amplitudes on each day measured.
(E) Appropriate data for each repeated trial (or session) showing acquisition and retention scores on the tests of learning and memory on each day measured.
(F) Time and cause of death (if appropriate); any neurological signs observed; a list of structures examined as well as the locations, nature, frequency, and extent of lesions; and brain weights.
(ii) The following data should also be provided, as appropriate:
(A) Inclusion of photomicrographs demonstrating typical examples of the type and extent of the neuropathological alterations observed is recommended.
(B) Any diagnoses derived from neurological signs and lesions, including naturally occurring diseases or conditions, should also be recorded.
(iii) Summary data for each treatment and control group must include:
(A) The number of animals at the start of the test.
(B) The body weight of the dams during gestation and lactation.
(C) Litter size and mean weight at birth.
(D) The number of animals showing each abnormal sign at each observation time.
(E) The percentage of animals showing each abnormal sign at each observation time.
(F) The mean and standard deviation for each continuous endpoint at each observation time. These will include body weight, motor activity counts, auditory startle responses, performance in learning and memory tests, regional brain weights and whole brain weights (both absolute and relative).
(G) The number of animals in which any lesion was found.
(H) The number of animals affected by each different type of lesion, the location, frequency and average grade of each type of lesion for each animal.
(I) The values of all morphometric measurements made for each animal listed by treatment group.
(3) Evaluation of data. An evaluation of test results must be made. The evaluation must include the relationship between the doses of the test substance and the presence or absence, incidence, and extent of any neurotoxic effect. The evaluation must include appropriate statistical analyses. The choice of analyses must consider tests appropriate to the experimental design and needed adjustments for multiple comparisons. The evaluation must include the relationship, if any, between observed neuropathological and behavioral alterations.
(f) References. For additional background information on this test guideline, the following references should be consulted. These references are available for inspection at the TSCA Nonconfidential Information Center, Rm. NE-B607, Environmental Protection Agency, 401 M St., SW., Washington, DC, 12 noon to 4 p.m., Monday through Friday, except legal holidays.
(1) Adams, J., Buelke-Sam, J., Kimmel, C.A., Nelson, C.J., Reiter, L.W., Sobotka, T.J., Tilson, H.A., and Nelson, B.K. Collaborative behavioral teratolgy study: Protocol design and testing procedures. Neurobehavioral Toxicology and Teratology 7:579-586 (1985).
(2) Bennett, H.S., Wyrick, A.D., Lee, S.W., and McNeil, J.H. Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technology 51:71-97 (1976).
(3) Bushnell, P.J. Effects of delay, intertrial interval, delay behavior and trimethyltin on spatial delayed response in rats. Neurotoxicology and Teratology 10:237-244 (1988).
(4) Campbell, B.A. and Haroutunian, V. Effects of age on long-term memory: Retention of fixed interval responding. Journal of Gerontology 36:338-341 (1981).
(5) Cory-Slechta, D.A., Weiss, B., and Cox, C. Delayed behavioral toxicity of lead with increasing exposure concentration. Toxicology and Applied Pharmacology 71:342-352 (1983).
(6) Di Sant Agnese, P. A. and De Mesy Jensen, K.L. Dibasic staining of large epoxy tissue sections and application to surgical pathology. American Journal of Clinical Pathology 81:25-29 (1984).
(7) U.S. Environmental Protection Agency. Neurotoxicity Screening Battery. In: Pesticide Assessment Guidelines, Subdivision F, Addendum 10. EPA 540/09-91-123. NTIS PB 91-154617 (1991).
(8) Friede, R. L. Developmental Neuropathology. Springer-Verlag, New York. pp. 1-23, 297-313, 326-351 (1975).
(9) Green, R.J. and Stanton, M.E. Differential ontogeny of working memory and reference memory in the Start Printed Page 78815rat. Behavioral Neuroscience 103:98-105 (1989).
(10) Ison, J.R. Reflex modification as an objective test for sensory processing following toxicant exposure. Neurobehavioral Toxicology and Teratology 6:437-445 (1984).
(11) Korenbrot, C.C., Huhtaniemi, I.T., and Weiner, R.I. Preputial separation as an external sign of pubertal development in the male rat. Biology of Reproduction 17:298-303 (1977).
(12) Krasnegor, N.A., Blass, E.M., Hofer, M.A., and Smotherman, W.P. (eds.) Perinatal Development: A Psychobiological Perspective. Academic Press, Orlando. pp.11-37, 145-167. (1987).
(13) Kucharski, D. and Spear, N.E. Conditioning of aversion to an odor paired with peripheral shock in the developing rat. Developmental Psychobiology 17:465-479 (1984).
(14) Luna, L. G. (editor). Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. (Third Edition). McGraw-Hill, New York. pp. 1-31 (1968).
(15) Miale, I. L. and Sidman, R.L. An autoradiographic analysis of histogenesis in the mouse cerebellum. Experimental Neurology. 4:277-296 (1961).
(16) Miller, D.B. and Eckerman, D.A. Learning and memory measures. In: Neurobehavioral Toxicology, Z. Annau (ed). Johns Hopkins University Press, Baltimore. pp. 94-149 (1986).
(17) Pender, M.P. A simple method for high resolution light microscopy of nervous tissue. Journal of Neuroscience Methods. 15:213-218 (1985).
(18) Ralis, H.M., Beesley, R.A., and Ralis, Z.A. Techniques in Neurohistology. Butterworths, London. pp. 57-145 (1973).
(19) Rodier, P.M. and Gramann, W.J. Morphologic effects of interference with cell proliferation in the early fetal period. Neurobehavioral Toxicology 1:129-135 (1979).
(20) Spear, N.E. and Campbell, B.A. (eds.) Ontogeny of Learning and Memory. Erlbaum, New Jersey. pp. 101-133, 157-224 (1979).
(21) Spencer, P.S., Bischoff, M.C., and Schaumburg, H.H. Neuropathological methods for the detection of neurotoxic disease. In: Experimental and Clinical Neurotoxicology. Spencer, P.S. and Schaumburg, H.H. (eds.). Williams and Wilkins, Baltimore. pp. 743-757 (1980).
(22) Suzuki, K. Special vulnerabilities of the developing nervous system to toxic substances. In: Experimental and Clinical Neurotoxicology. Spencer, P.S. and Schaumburg, H.H. (eds.). Williams and Wilkins, Baltimore. pp. 48-61 (1980). (23) Luna, L.G. (ed.). Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. (Third Edition). McGraw-Hill, New York. pp. 32-46 (1968).
End Supplemental InformationTSCA metabolism and pharmacokinetics(a) Scope. (1) This section is intended to meet the testing requirements under section 4 of the Toxic Substances Control Act (TSCA). (1) Testing of the disposition of a test substance is designed to obtain adequate information on its absorption, distribution, biotransformation, and excretion and to aid in understanding the mechanism of toxicity. Basic pharmacokinetic parameters determined from these studies will also provide information on the potential for accumulation of the test substance in tissues and/or organs and the potential for induction of biotransformation as a result of exposure to the test substance. These data can be used to assess the adequacy and relevance of the extrapolation of animal toxicity data (particularly chronic toxicity and/or carcinogenicity data) to human risk assessment.
(2) Metabolism data can also be used to assist in determining whether animal toxicity studies have adequately addressed any toxicity concerns arising from exposure to plant metabolites, and in the setting of tolerances, if any, for those metabolites in raw agricultural commodities.
(b) Source. The source material used in developing this TSCA test guideline is the Office of Prevention, Pesticides and Toxic Substances (OPPTS) harmonized test guideline 870.7485 (August 1998, final guideline). This source is available at the address in paragraph (h) of this section.
(c) Definitions. The following definitions apply to this section.
Metabolism (biotransformation) is the sum of the processes by which a foreign chemical is subjected to chemical change by living organisms.
LOEL is the lowest observable effects level.
NOEL is the no observable effects level.
Pharmacokinetics is the quantitation and determination of the time course and dose dependency of the absorption, distribution, biotransformation, and excretion of chemicals.
(d) Good laboratory practice standards. The pharmacokinetics and metabolism tests outlined in this guideline must conform to the laboratory practices stipulated in 40 CFR Part 792—Good Laboratory Practice Standards.
(e) Test Procedures. Test procedures presented below utilize a tier system to minimize the use of resources and to allow flexibility in the conduct of metabolism studies. The proposed tier system consists of a basic data set (Tier 1) and additional studies (Tier 2). These additional studies may be requested based upon the existing toxicology data base and/or the results of Tier 1 testing which are found to impact upon the risk assessment process. For Tier 1 testing, the oral route will typically be required; however, if the use pattern results in other types of exposure, other routes (dermal and/or inhalation) may be required for initial testing of the disposition of a chemical substance. The registrant should justify the route of exposure to the Agency. Complete descriptions of the test procedures for these other routes of exposure can be found in paragraph (i) of this section. Except in unusual circumstances, the tiered approach to metabolism testing should apply to all listed routes of exposure.
(1) Pilot studies. The use of pilot studies is recommended and encouraged for the selection of experimental conditions for the pharmacokinetics and metabolism studies (mass balance, analytical procedures, dose-finding, excretion of CO2, etc.).
(2) Animal selection—(i) Species. The rat must normally be used for testing because it has been used extensively for metabolic and toxicological studies. The use of other or additional species may be required if critical toxicology studies demonstrate evidence of significant toxicity in these species or if metabolism is shown to be more relevant to humans in the test species.
(ii) Strain. Adult animals of the strain used or proposed to be used for the determination of adverse health effects associated with the test substance.
(3) Material to be tested—(i) Test substance. (A) A radiolabeled test substance using14 C should be used for all material balance and metabolite identification aspects of the study. Other radioactive and stable isotopes may be used, particularly if the element is responsible for or is a part of the toxic portion of the compound. If it can be demonstrated that the material balance and metabolite identification requirements can be met using unlabeled test substance, then radiolabeled compound need not be used. If possible, the radiolabel should be located in a core portion of the molecule which is metabolically stable (it is not exchangeable, is not removed metabolically as CO2, and does not become part of the one-carbon pool of the organism). Labeling of multiple sites of the molecule may be necessary to Start Printed Page 78816follow the metabolic fate of the compound.
(B) The label should follow the test compound and/or its major metabolites until excreted. The radiopurity of the radioactive test substance shall be the highest attainable for a particular test substance (ideally it should be greater than 95%) and reasonable effort should be made to identify impurities present at or above 2%. The purity, along with the identity of major impurities which have been identified, shall be reported. For other segments of the study, nonradioactive test substance may be used if it can be demonstrated that the analytical specificity and sensitivity of the method used with nonradioactive test substance is equal to or greater than that which could be obtained with the radiolabeled test substance. The radioactive and nonradioactive test substances shall be analyzed using an appropriate method to establish purity and identity. Additional guidance will be provided in chemical specific test rules to assist in the definition and specifications of test substances composed of mixtures and methods for determination of purity.
(ii) Administration of test substance. Test substance should be dissolved or suspended homogeneously in a vehicle usually employed for acute administration. A rationale for the choice of vehicle should be provided. The customary method of administration will be by oral gavage; however, administration by gelatin capsule or as a dietary mixture may be advantageous in specific situations. Verification of the actual dose administered to each animal should be provided.
(4) Tier testing. (i) The multiplicity of metabolic parameters that impact the outcome of toxicological evaluations preclude the use of a universal study design for routine toxicological evaluation of a test substance. The usefulness of a particular study design depends upon the biological activity of a compound and circumstances of exposure. For these reasons, a tiered system is proposed for evaluation of the metabolism/kinetic properties of a test substance.
(ii) The first tier data set is a definitive study by the appropriate route of exposure conducted in male rats to determine the routes and rate of excretion and to identify excreted metabolites. First tier data will also provide basic information for additional testing (Tier 2) if such testing is considered necessary. In the majority of cases, Tier 1 data are expected to satisfy regulatory requirements for biotransformation and pharmacokinetic data on test chemicals.
(iii) Second tier testing describes a variety of metabolism/kinetic experiments which address specific questions based on the existing toxicology data base and/or those results of Tier 1 testing impacting significantly on the risk assessment process. For conduct of these studies, individualized protocols may be necessary. Protocols for these studies, if required, can be developed as a cooperative effort between Agency and industry scientists.
(f) Tier 1 data requirements (minimum data set). At this initial level of testing, biotransformation and pharmacokinetic data from a single low dose group will be required. This study will determine the rate and routes of excretion and the type of metabolites generated.
(1) Number and sex of animals. A minimum of four male young adult animals must be used for Tier 1 testing. The use of both sexes may be required in cases where there is evidence to support significant sex-related differences in toxicity.
(2) Dose selection. (i) A single dose is required for each route of exposure. The dose should be nontoxic, but high enough to allow for metabolite identification in excreta. If no other toxicity data are available for selection of the low dose, a dose identified as a fraction of the LD50 (as determined from acute toxicity studies) may be used. The magnitude of the dose used in Tier 1 studies should be justified in the final report.
(ii) For test substances of low toxicity a maximum dose of 1,000 mg/kg should be used; chemical-specific considerations may necessitate a higher maximum dose and will be addressed in specific test rules.
(3) Measurements—(i) Excretion. (A) Data obtained from this section (percent recovery of administered dose from urine, feces, and expired air) will be used to determine the rate and extent of excretion of test chemical, to assist in establishing mass balance, and will be used in conjunction with pharmacokinetic parameters to determine the extent of absorption. The quantities of radioactivity eliminated in the urine, feces, and expired air shall be determined separately at appropriate time intervals.
(B) If a pilot study has shown that no significant amount of radioactivity is excreted in expired air, then expired air need not be collected in the definitive study.
(C) Each animal must be placed in a separate metabolic unit for collection of excreta (urine, feces and expired air). At the end of each collection period, the metabolic units must be rinsed with appropriate solvent to ensure maximum recovery of radiolabel. Excreta collection must be terminated at 7 days, or after at least 90% of the administered dose has been recovered, whichever occurs first. The total quantities of radioactivity in urine must be determined at 6, 12, and 24 hours on day 1 of collection, and daily thereafter until study termination, unless pilot studies suggest alternate or additional time points for collection. The total quantities of radioactivity in feces should be determined on a daily basis beginning at 24 hours post-dose, and daily thereafter until study termination. The collection of CO2 and other volatile materials may be discontinued when less than 1% of the administered dose is found in the exhaled air during a 24-hour collection period.
(ii) Tissue distribution. At the termination of the Tier 1 study, the following tissues should be collected and stored frozen: Liver, fat, gastrointestinal tract, kidney, spleen, whole blood, and residual carcass. If it is determined that a significant amount of the administered dose is unaccounted for in the excreta, then data on the percent of the total (free and bound) radioactive dose in these tissues as well as residual carcass will be requested. Additional tissues must be included if there is evidence of target organ toxicity from subchronic or chronic toxicity studies. For other routes of exposure, specific tissues may also be required, such as lungs in inhalation studies and skin in dermal studies. Certain techniques currently at various stages of development, e.g., quantitative whole-body autoradiography, may prove useful in determining if a test substance concentrates in certain organs or in determining a specific pattern of distribution within a given tissue. The use of such techniques is encouraged, but not required, and may be employed to limit the number of tissues collected to those shown to contain a measurable amount of radioactivity.
(iii) Metabolism. Excreta must be collected for identification and quantitation of unchanged test substance and metabolites as described in paragraph (f)(3)(i) of this section. Pooling of excreta to facilitate metabolite identification within a given dose group is acceptable. Profiling of metabolites from each time period is recommended. However, if lack of sample and/or radioactivity precludes this, pooling of urine as well as pooling of feces across several time points is acceptable. Appropriate qualitative and quantitative methods must be used to Start Printed Page 78817assay urine, feces, and expired air from treated animals. Reasonable efforts should be made to identify all metabolites present at 5% or greater of the administered dose and to provide a metabolic scheme for the test chemical. Compounds which have been characterized in excreta as comprising 5% or greater of the administered dose should be identified. If identification at this level is not possible, a justification/explanation should be provided in the final report. Identification of metabolites representing less than 5% of the administered dose might be requested if such data are needed for risk assessment of the test chemical. Structural confirmation should be provided whenever possible. Validation of the methods used in metabolite identification should be included.
(g) Tier 2 data requirements. Studies at the Tier 2 level are designed to answer questions about the disposition of test chemicals based on the existing toxicology data base and/or results of Tier 1 testing which may have a significant impact on the risk assessment for the test chemical. Such studies may address questions regarding absorption, persistence, or distribution of the test chemical, or a definitive alteration in the metabolic profile occurring with dose which may be of toxicological concern. At the Tier 2 level, only those studies which address a specific concern are required, and if required must be conducted according to mutual agreement between the registrant and the Agency. Flexibility will be allowed in the design of specific experiments as warranted by technological advances in this field.
(1) Absorption. (i) If the extent of absorption cannot be established from Tier 1 studies, or where greater than 20% of the administered dose is present in feces, a study to determine the extent of absorption will be required. This can be accomplished either through intravenous administration of test material and measurement of radioactivity in excreta or after oral administration of test material and measurement of radioactivity in bile.
(ii) For the intravenous study, a single dose (not to exceed the oral dose used in Tier 1) of test chemical using an appropriate vehicle should be administered in a suitable volume (e.g., 1 mL/kg) at a suitable site to at least three male rats (both sexes might be used if warranted). The disposition of the test chemical should be monitored for oral dosing as outlined in paragraph (f)(3)(i) of this section. Metabolite identification will not be required for this study.
(iii) If a biliary excretion study is chosen the oral route of administration may be requested. In this study, the bile ducts of at least three male rats (or of both sexes, if warranted) should be appropriately cannulated and a single dose of the test chemical should be administered to these rats. Following administration of the test chemical, excretion of radioactivity in bile should be monitored as long as necessary to determine if a significant percentage of the administered dose is excreted via this route.
(2) Tissue distribution time course. (i) A time course of tissue distribution in selected tissues may be required to aid in the determination of a possible mode of toxic action. This concern may arise from evidence of extended half-life or possible accumulation of radioactivity in specific tissues. The selection of tissues for this type of study will be based upon available evidence of target organ toxicity and/or carcinogenicity, and the number of time points required will be based upon pharmacokinetic information obtained from Tier 1 data. Flexibility will be allowed in the selection of time points to be studied.
(ii) For this type of study, three rats per time point will be administered an appropriate oral dose of test chemical, and the time course of distribution monitored in selected tissues. Only one sex may be required, unless target organ toxicity is observed in sex-specific organs. Assessment of tissue distribution will be made using appropriate techniques for assessment of total amount distributed to tissue and for assessment of metabolite distribution.
(3) Plasma kinetics. The purpose of this experiment is to obtain estimates of basic pharmacokinetic parameters (half-life, volume of distribution, absorption rate constant, area under the curve) for the test substance. Kinetic data may be required if the data can be used to resolve issues about bioavailability and to clarify whether clearance is saturated in a dose-dependent fashion. For this experiment a minimum of three rats per group is required. At least two doses will be required, usually the NOEL and LOEL from the critical toxicology study. Following administration of test substance, samples should be obtained from each animal at suitable time points appropriate sampling methodology. Total radioactivity present (or total amount of chemical, for nonradioactive materials) should be analyzed in whole blood and plasma using appropriate methods, and the blood/plasma ratio should be calculated.
(4) Induction. (i) Studies addressing possible induction of biotransformation may be requested under one or more of the following conditions:
(A) Available evidence indicates a relationship between induced metabolism and enhanced toxicity.
(B) The available toxicity data indicate a nonlinear relationship between dose and metabolism.
(C) The results of Tier 1 metabolite identification studies show identification of a potentially toxic metabolite.
(D) Induction can plausibly be invoked as a factor in such effects where status may depend on the level of inducible enzymes present. Several in vivo and in vitro methods are available for assessment of enzyme induction, and the experiments which best address the issue at hand can be determined between Agency and industry scientists. If induction is demonstrated, the relationship of this phenomenon to toxicity observed from subchronic and/or chronic toxicity studies will need to be addressed.
(ii) [Reserved]
(iii) If toxicologically significant alterations in the metabolic profile of the test chemical are observed through either in vitro or in vivo experiments, characterization of the enzyme(s) involved (for example, Phase I enzymes such as isozymes of the Cytochrome P450-dependent mono-oxygenase system, Phase II enzymes such as isozymes of sulfotransferase or uridine diphosphate glucuronosyl transferase, or any other relevant enzymes) may be requested. This information will help establish the relevance of the involved enzyme(s) to human risk, as it is known that certain isozymes are present in animal species which are not present in humans, and vice versa.
(5) Physiologically-based modeling. Traditional methods of modeling have been used to determine kinetic parameters associated with drug and xenobiotic disposition, but have assumed a purely mathematical construct of mammalian organisms in their operation. On the other hand, more recent models which take into account the physiological processes of the animal have been used with success in defining biological determinants of chemical disposition as well as the relationship between tissue dose and tissue response. These so-called physiologically-based models, also allow for cross-species extrapolation which is often necessary in the risk-assessment process. The use of physiologically-based modeling as an experimental tool for addressing specific issues related to biotransformation and pharmacokinetics of a test substance is encouraged. Start Printed Page 78818Information as derived from physiologically-based modeling experiments may aid in the comparison of biotransformation and pharmacokinetics of a test substance between animal species and humans, and in the assessment of risk under specific exposure conditions. At the discretion of the Agency, or by mutual agreement, results of physiologically based pharmacokinetic (PBPK) studies with parent compound may be submitted in lieu of other studies, if it is determined that such data would provide adequate information to satisfy this guideline.
(h) Reporting of study results. In addition to the reporting requirements specified under EPA Good Laboratory Practice Standards at 40 CFR part 792, subpart J, the completed study (Tier 1 or Tier 2) should be presented in the following format:
(1) Title/cover page. Title page and additional requirements (requirements for data submission, good laboratory practice, statements of data confidentiality claims and quality assurance) if relevant to the study report, should precede the content of the study formatted below. These requirements are to be found in 40 CFR parts 790, 792, and 799.
(2) Table of contents. A concise listing must precede the body of the report, containing all essential elements of the study and the page and table number where the element is located in the final report of the study. Essential elements of the table of contents should include a summary, an introduction, the materials and methods section, results, discussion/conclusions, references, tables, figures, appendices, and key subsections as deemed appropriate. The table of contents should include the page number of each of these elements.
(3) Body of the report. The body of the report must include information required under this section, organized into sections and paragraphs as follows:
(i) Summary. This section of the study report must contain a summary and analysis of the test results and a statement of the conclusions drawn from the analysis. This section should highlight the nature and magnitude of metabolites, tissue residue, rate of clearance, bioaccumulation potential, sex differences, etc. The summary should be presented in sufficient detail to permit independent evaluation of the findings.
(ii) Introduction. This section of the report should include the objectives of the study, guideline references, regulatory history, if any, and a rationale.
(iii) Materials and methods. This section of the report must include detailed descriptions of all elements including:
(A) Test substance. (1) This section should include identification of the test substance—chemical name, molecular structure, qualitative and quantitative determination of its chemical composition, and type and quantities of any impurities whenever possible.
(2) This section should also include information on physical properties including physical state, color, gross solubility and/or partition coefficient, and stability.
(3) The type or description of any vehicle, diluents, suspending agents, and emulsifiers or other materials used in administering the test substance should be stated.
(4) If the test substance is radiolabeled, information on the following should be included in this subsection: The type of radionuclide, position of label, specific activity, and radiopurity.
(B) Test animals. This section should include information on the test animals, including: Species, strain, age at study initiation, sex, body weight, health status, and animal husbandry.
(C) Methods. This subsection should include details of the study design and methodology used. It should include a description of:
(1) How the dosing solution was prepared and the type of solvent, if any, used.
(2) Number of treatment groups and number of animals per group.
(3) Dosage levels and volume.
(4) Route of administration.
(5) Frequency of dosing.
(6) Fasting period (if used).
(7) Total radioactivity per animal.
(8) Animal handling.
(9) Sample collection.
(10) Sample handling.
(11) Analytical methods used for separation.
(12) Quantitation and identification of metabolites.
(13) Other experimental measurements and procedures employed (including validation of test methods for metabolite analysis).
(D) Statistical analysis. If statistical analysis is used to analyze the study findings, then sufficient information on the method of analysis and the computer program employed should be included so that an independent reviewer/statistician can reevaluate and reconstruct the analysis. Presentation of models should include a full description of the model to allow independent reconstruction and validation of the model.
(iv) Results. All data should be summarized and tabulated with appropriate statistical evaluation and placed in the text of this section. Radioactivity counting data should be summarized and presented as appropriate for the study, typically as disintegrations per minute and microgram or milligram equivalents, although other units may be used. Graphic illustrations of the findings, reproduction of representative chromatographic and spectrometric data, and proposed metabolic pathways and molecular structure of metabolites should be included in this section. In addition the following information is to be included in this section if applicable:
(A) Justification for modification of exposure conditions, if applicable.
(B) Justification for selection of dose levels for pharmacokinetic and metabolism studies.
(C) Description of pilot studies used in the experimental design of the pharmacokinetic and metabolism studies, if applicable.
(D) Quantity and percent recovery of radioactivity in urine, feces, and expired air, as appropriate. For dermal studies, include recovery data for treated skin, skin washes, and residual radioactivity in the covering apparatus and metabolic unit as well as results of the dermal washing study.
(E) Tissue distribution reported as percent of administered dose and microgram equivalents per gram of tissue.
(F) Material balance developed from each study involving the assay of body tissues and excreta.
(G) Plasma levels and pharmacokinetic parameters after administration by the relevant routes of exposure.
(H) Rate and extent of absorption of the test substance after administration by the relevant routes of exposure.
(I) Quantities of the test substance and metabolites (reported as percent of the administered dose) collected in excreta.
(J) Individual animal data.
(v) Discussion and conclusions. (A) In this section the author(s) should:
(1) Provide a plausible explanation of the metabolic pathway for the test chemical.
(2) Emphasize species and sex differences whenever possible.
(3) Discuss the nature and magnitude of metabolites, rates of clearance, bioaccumulation potential, and level of tissue residues as appropriate.
(B) The author(s) should be able to derive a concise conclusion that can be supported by the findings of the study.
(vi) Optional sections. The authors may include additional sections such as appendices, bibliography, tables, etc. Start Printed Page 78819
(i) Alternate routes of exposure for Tier 1 testing—(1) Dermal—(i) Dermal treatment. One (or more if needed) dose levels of the test substance must be used in the dermal portion of the study. The low dose level should be selected in accordance with paragraph (f)(2) of this section. The dermal doses must be dissolved, if necessary, in a suitable vehicle and applied in a volume adequate to deliver the doses. Shortly before testing, fur is to be clipped from the dorsal area of the trunk of the test animals. Shaving may be employed, but it should be carried out approximately 24 hour before the test. When clipping or shaving the fur, care should be taken to avoid abrading the skin, which could alter its permeability. Approximately 10% of the body surface should be cleared for application of the test substance. With highly toxic substances, the surface area covered may be less than approximately 10%, but as much of the area as possible is to be covered with a thin and uniform film. The same nominal treatment surface area must be used for all dermal test groups. The dosed areas are to be protected with a suitable covering which is secured in place. The animals must be housed separately.
(ii) Dermal washing study. (A) A washing experiment must be conducted to assess the removal of the applied dose of the test substance by washing the treated skin area with a mild soap and water. A single dose must be applied to two animals in accordance with paragraph (f)(2) of this section. After application (2 to 5 minutes) the treated areas of the animals must be washed with a mild soap and water. The amounts of test substance recovered in the washes must be determined to assess the effectiveness of removal by washing.
(B) Unless precluded by corrosiveness, the test substance must be applied and kept on the skin for a minimum of 6 hours. At the time of removal of the covering, the treated area must be washed following the procedure as outlined in the dermal washing study. Both the covering and the washes must be analyzed for residual test substance. At the termination of the studies, each animal must be sacrificed and the treated skin removed. An appropriate section of treated skin must be analyzed to determine residual radioactivity.
(2) Inhalation. A single (or more if needed) concentration of test substance must be used in this portion of the study. The concentration should be selected in accordance with paragraph (f)(2) of this section. Inhalation treatments are to be conducted using a “nose-cone” or “head-only” apparatus to prevent absorption by alternate routes of exposure. If other inhalation exposure conditions are proposed for use in a chemical-specific test rule, justification for the modification must be documented. A single exposure over a defined period must be used for each group—a typical exposure is 4-6 hours.
Footnotes
1. For a number of measurements in serum and plasma, most notably for glucose, overnight fasting would be preferable. The major reason for this preference is that the increased variability which would inevitably result from non-fasting, would tend to mask more subtle effects and make interpretation difficult. On the other hand, however, overnight fasting may interfere with the general metabolism of the animals and, particularly in feeding studies, may disturb the daily exposure to the test substance. If overnight fasting is adopted, clinical biochemical determinations should be performed after the conduct of functional observations in week 4 of the study.
Back to Citation1. For a number of measurements in serum and plasma, most notably for glucose, overnight fasting would be preferable. The major reason for this preference is that the increased variability which would inevitably result from non-fasting, would tend to mask more subtle effects and make interpretation difficult. On the other hand, however, overnight fasting may interfere with the general metabolism of the (pregnant) animals, disturbs lactation and nursing behaviour, and, particularly in feeding studies, may disturb the daily exposure to the test substance. If overnight fasting is adopted, clinical biochemical determinations should be performed after the conduct of functional observations in week 4 of the study.
Back to Citation[FR Doc. 00-31728 Filed 12-14-00; 8:45 am]
BILLING CODE 6560-50-F
Document Information
- Effective Date:
- 12/15/2000
- Published:
- 12/15/2000
- Department:
- Environmental Protection Agency
- Entry Type:
- Rule
- Action:
- Final rule.
- Document Number:
- 00-31728
- Dates:
- This rule is effective on December 15, 2000.
- Pages:
- 78745-78819 (75 pages)
- Docket Numbers:
- OPPTS-42211, FRL-6551-2
- RINs:
- 2070-AD16: High Production Volume (HPV) Chemicals; 1st Group of Chemicals
- RIN Links:
- https://www.federalregister.gov/regulations/2070-AD16/high-production-volume-hpv-chemicals-1st-group-of-chemicals
- Topics:
- Chemicals, Environmental protection, Hazardous substances, Health, Reporting and recordkeeping requirements
- PDF File:
- 00-31728.pdf
- CFR: (17)
- 40 CFR 799.6755
- 40 CFR 799.6756
- 40 CFR 799.6784
- 40 CFR 799.6786
- 40 CFR 799.9110
- More ...