2017-27317. Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use
-
Start Preamble
Start Printed Page 60474
AGENCY:
Food and Drug Administration, HHS.
ACTION:
Final rule.
SUMMARY:
The Food and Drug Administration (FDA, the Agency, or we) is issuing this final rule establishing that certain active ingredients used in nonprescription (also known as over-the-counter or OTC) antiseptic products intended for use by health care professionals in a hospital setting or other health care situations outside the hospital are not generally recognized as safe and effective (GRAS/GRAE). FDA is issuing this final rule after considering the recommendations of the Nonprescription Drugs Advisory Committee (NDAC); public comments on the Agency's notices of proposed rulemaking; and all data and information on OTC health care antiseptic products that have come to the Agency's attention. This final rule finalizes the 1994 tentative final monograph (TFM) for OTC health care antiseptic drug products that published in the Federal Register of June 17, 1994 (the 1994 TFM) as amended by the proposed rule published in the Federal Register (FR) of May 1, 2015 (2015 Health Care Antiseptic Proposed Rule (PR)).
DATES:
This rule is effective December 20, 2018.
ADDRESSES:
For access to the docket to read background documents or the electronic and written/paper comments received, go to https://www.regulations.gov and insert the docket number found in brackets in the heading of this final rule, into the “Search” box and follow the prompts, and/or go to the Dockets Management Staff, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852.
Start Further InfoFOR FURTHER INFORMATION CONTACT:
Michelle M. Jackson, Center for Drug Evaluation and Research, Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 22, Rm. 5420, Silver Spring, MD 20993-0002, 301-796-0923.
End Further Info End Preamble Start Supplemental InformationSUPPLEMENTARY INFORMATION:
Table of Contents
I. Executive Summary
A. Purpose of the Final Rule
B. Summary of the Major Provisions of the Final Rule
C. Costs and Benefits
II. Table of Abbreviations and Acronyms Commonly Used in This Document
III. Introduction
A. Terminology Used in the OTC Drug Review Regulations
B. Topical Antiseptics
C. This Final Rule Covers Only Health Care Antiseptics
IV. Background
A. Significant Rulemakings Relevant to This Final Rule
B. Public Meetings Relevant to This Final Rule
C. Scope of This Final Rule
D. Eligibility for the OTC Drug Review
V. Comments on the Proposed Rule and FDA Response
A. Introduction
B. General Comments on the Proposed Rule and FDA Response
C. Comments on Eligibility of Active Ingredients and FDA Response
D. Comments on Effectiveness and FDA Response
E. Comments on Safety and FDA Response
F. Comments on the Preliminary Regulatory Impact Analysis and FDA Response
VI. Ingredients Not Generally Recognized as Safe and Effective
VII. Compliance Date
VIII. Summary of Regulatory Impact Analysis
A. Introduction
B. Summary of Costs and Benefits
IX. Paperwork Reduction Act of 1995
X. Analysis of Environmental Impact
XI. Federalism
XII. References
I. Executive Summary
A. Purpose of the Final Rule
This final rule finalizes the 2015 Health Care Antiseptic PR. This final rule applies to health care antiseptic products that are intended for use by health care professionals in a hospital setting or other health care situations outside the hospital. Health care antiseptic products include health care personnel hand washes, health care personnel hand rubs, surgical hand scrubs, surgical hand rubs, and patient antiseptic skin preparations (i.e., patient preoperative and preinjection skin preparations).
In response to several requests submitted to the 2015 Health Care Antiseptic PR, FDA has deferred further rulemaking on six active ingredients used in OTC health care antiseptic products to allow for the development and submission to the record of new safety and effectiveness data for these ingredients. The deferred active ingredients are benzalkonium chloride, benzethonium chloride, chloroxylenol, alcohol (also referred to as ethanol or ethyl alcohol), isopropyl alcohol, and povidone-iodine. Accordingly, FDA does not make a GRAS/GRAE determination in this final rule for these six active ingredients for use as OTC health care antiseptics. The monograph or nonmonograph status of these six ingredients will be addressed, either after completion and analysis of ongoing studies to address the safety and effectiveness data gaps of these ingredients or at a later date, if these studies are not completed.
This rulemaking finalizes the nonmonograph status of the remaining 24 active ingredients intended for use in health care antiseptics identified in the 2015 Health Care Antiseptic PR. No additional data were submitted to support monograph conditions for these 24 health care antiseptic active ingredients. Therefore, this rule finalizes the 2015 Health Care Antiseptic PR and finds that 24 health care antiseptic active ingredients are not GRAS/GRAE for use as OTC health care antiseptics. Accordingly, OTC health care antiseptic drugs containing any of these 24 active ingredients are new drugs under section 201(p) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 321(p)) for which approved applications under section 505 of the FD&C Act (21 U.S.C. 355) and part 314 (21 CFR 314) of the regulations are required for marketing and may be misbranded under section 502 of the FD&C Act (21 U.S.C. 352).
This final rule covers only OTC health care antiseptics that are intended for use by health care professionals in a hospital setting or other health care situations outside the hospital. This final rule does not cover consumer antiseptic washes (78 FR 76444, 81 FR 61106); consumer antiseptic rubs (81 FR 42912); antiseptics identified as “first aid antiseptics” in the 1991 First Aid tentative final monograph (TFM) (56 FR 33644); or antiseptics used by the food industry.
B. Summary of the Major Provisions of the Final Rule
1. Safety
Several important scientific developments that affect the safety evaluation of OTC health care antiseptic active ingredients have occurred since FDA's 1994 safety evaluation. Improved analytical methods now exist that can detect and more accurately measure these active ingredients at lower levels in the bloodstream and tissue. Consequently, new data suggest that the Start Printed Page 60475systemic exposure to these active ingredients is higher than previously thought, and new information about the potential risks from systemic absorption and long-term exposure is now available. New safety information also suggests that widespread antiseptic use could have an impact on the development of bacterial resistance. To support a classification of generally recognized as safe (GRAS) for health care antiseptic active ingredients, we proposed that additional data were needed to demonstrate that those ingredients meet current safety standards (80 FR 25166 at 25179 to 25195).
The minimum data needed to demonstrate safety for all health care antiseptic active ingredients fall into four broad categories: (1) Human safety studies described in current FDA guidance (e.g., maximal usage trial or “MUsT”); (2) nonclinical safety studies described in current FDA guidance (e.g., developmental and reproductive toxicity studies and carcinogenicity studies); (3) data to characterize potential hormonal effects; and (4) data to evaluate the development of antimicrobial resistance.
We have considered the recommendations from the public meetings held by the Agency on antiseptics (see section IV.B, table 2) and evaluated the available literature, as well as the data, the comments, and other information that were submitted to the rulemaking on the safety of the 24 non-deferred health care antiseptic active ingredients addressed in this final rule. The available information and published data for these 24 active ingredients considered in this final rule are insufficient to establish the safety of these active ingredients for use in health care antiseptic products. No additional data were provided for these 24 ingredients. Consequently, the available data do not support a GRAS determination for the OTC non-deferred health care antiseptic active ingredients addressed in this final rule.
2. Effectiveness
A determination that an active ingredient is GRAS/GRAE for a particular intended use requires a benefit-to-risk assessment for the drug for that use. New information on potential risks posed by the increased use of certain health care antiseptics in clinical practice, as well as input from the 2005 NDAC, prompted us to reevaluate the data needed to determine whether health care antiseptic active ingredients are generally recognized as effective (GRAE). We continued to propose the use of surrogate endpoints (bacterial log reductions) as a demonstration of effectiveness for health care antiseptics combined with in vitro testing to characterize the antimicrobial activity of the active ingredient (80 FR 25166).
We have considered the recommendations from the public meetings held by the Agency on antiseptics (see section IV.B, table 2) and evaluated the available literature, as well as the data, the comments, and other information that were submitted to the rulemaking on the effectiveness of the 24 non-deferred health care antiseptic active ingredients addressed in this final rule. Since the publication of the 2015 Health Care Antiseptic PR, no new data or information was submitted on the effectiveness of these 24 non-deferred health care antiseptic active ingredients. Consequently, there is insufficient data to support a GRAE determination for these ingredients.
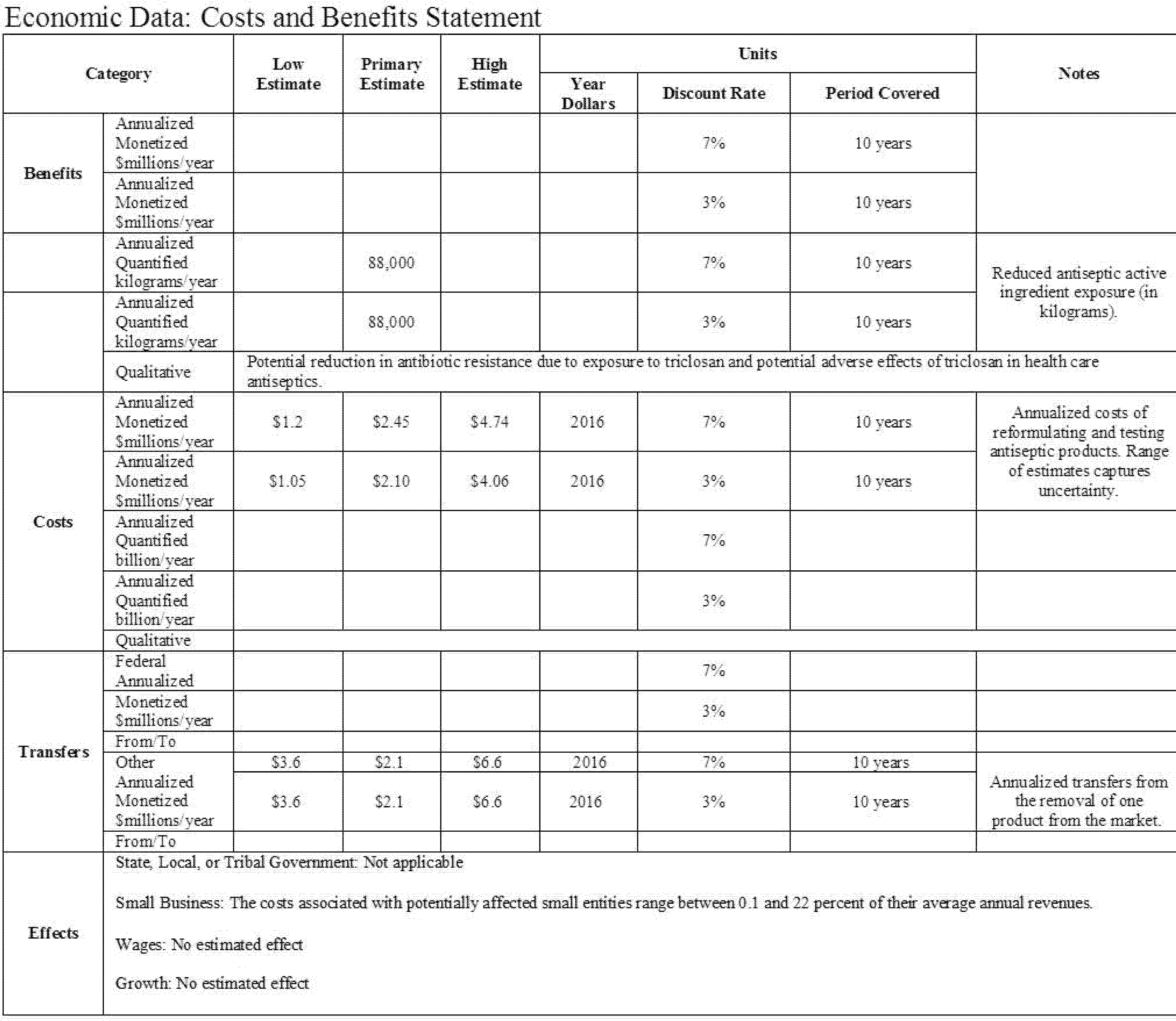
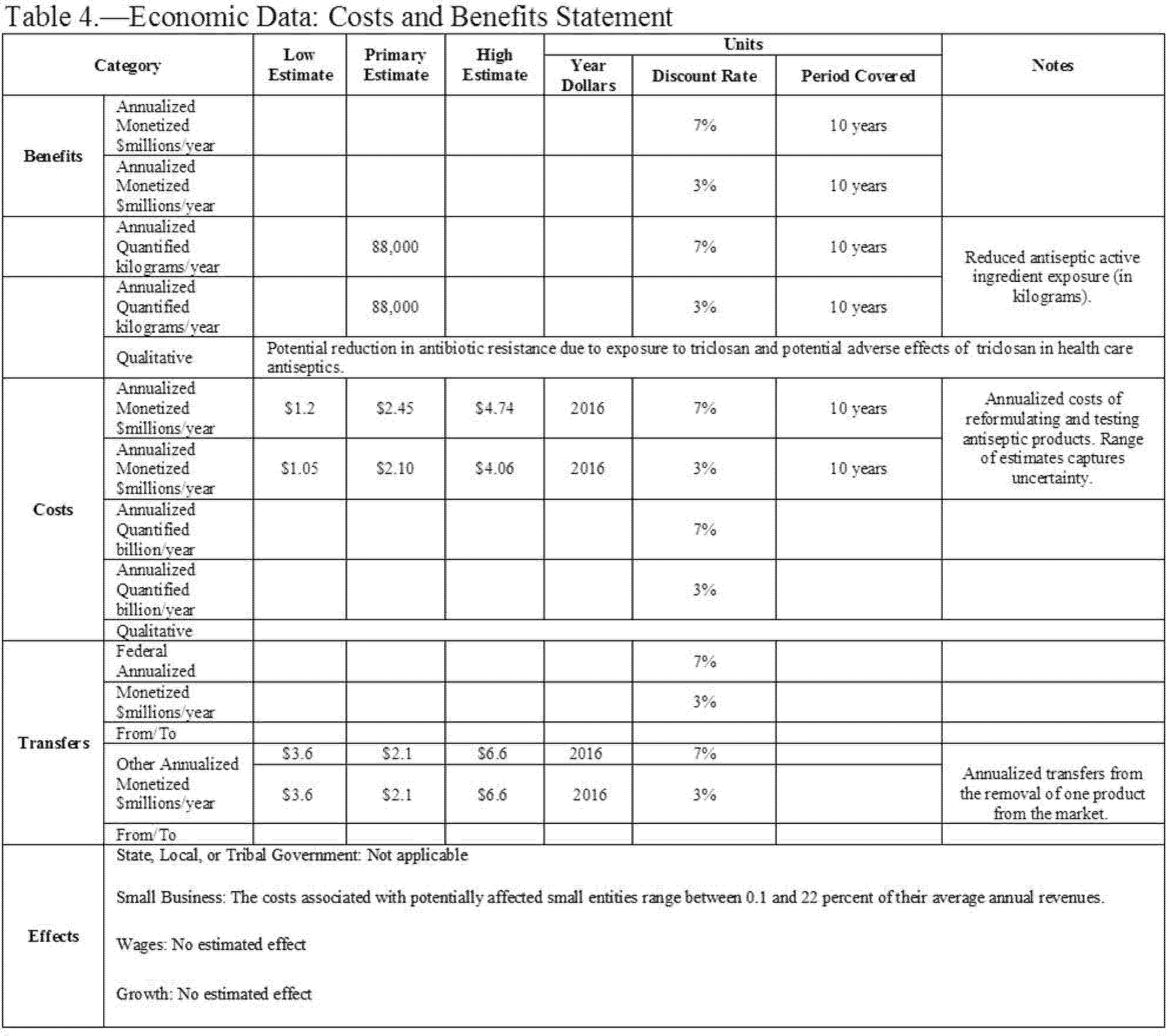
C. Costs and Benefits
This rule establishes that 24 eligible active ingredients are not generally recognized as safe and effective for use in nonprescription (also referred to as over-the-counter or OTC) health care antiseptics. However, data from the FDA drug product registration database suggest that only one of these 24 ingredients is found in OTC health care antiseptic products currently marketed pursuant to the TFM: Triclosan. Regulatory action is being deferred on six active ingredients that were included in the health care antiseptic proposed rule: Benzalkonium chloride, benzethonium chloride, chloroxylenol, ethyl alcohol, isopropyl alcohol, and povidone-iodine. This final rule also addresses comments on the eligibility of three active ingredients—alcohol (ethyl alcohol), benzethonium chloride, and chlorhexidine gluconate—and finds that these three active ingredients are ineligible for evaluation under the OTC Drug Review for certain health care antiseptic uses because these active ingredients were not included in health care antiseptic products marketed for the specified indications prior to May 1972. To our knowledge, there is only one ineligible product currently on the market, an alcohol-containing surgical hand scrub, which is affected by this rule.
Benefits are quantified as the volume reduction in exposure to triclosan found in health care antiseptic products affected by the rule, but these benefits are not monetized. Annual benefits are estimated to be a reduction in exposure of 88,000 kilograms (kg) of triclosan per year.
Costs are calculated as the one-time costs associated with reformulating health care antiseptic products containing the active ingredient triclosan and relabeling reformulated products. We believe that the alcohol-containing surgical hand scrub that is affected by this rule is likely to be removed from the market. We categorize the associated loss of sales revenue as a transfer from one manufacturer to another and not a cost, because we assume that the supply of other, highly substitutable, products is highly elastic.
Annualizing the one-time costs over a 10-year period, we estimate total annualized costs to range from $1.1 to $4.1 million at a 3 percent discount rate, and from $1.2 to $4.7 million at a 7 percent discount rate. The present value of total costs ranges from $9.0 to $34.6 million at a 3 percent discount rate, and from $8.7 to $29.6 million at a 7 percent discount rate.
In this final rule, small entities will bear costs to the extent that they must reformulate and re-label any health care antiseptic containing triclosan that they produce. The average cost to small firms of implementing the requirements of this final rule is estimated to be $213,176 per firm. The costs of the changes, along with the small number of firms affected, implies that this burden would not be significant, so we certify that this final rule will not have a significant economic impact on a substantial number of small entities. This analysis, together with other relevant sections of this document, serves as the Regulatory Flexibility Analysis, as required under the Regulatory Flexibility Act.
The full discussion of economic impacts is available in docket FDA-2015-N-0101 and at https://www.fda.gov/AboutFDA/ReportsManualsForms/Reports/EconomicAnalyses/default.htm.
Start Printed Page 60476Executive Order 13771 Summary Table
[In $ millions 2016 dollars, over an infinite time horizon]
Primary (7%) Lower bound (7%) Upper bound (7%) Present Value of Costs $17.19 $8.68 $29.47 Present Value of Cost Savings Present Value of Net Costs 17.19 8.68 29.47 Annualized Costs 1.20 0.61 2.06 Annualized Cost Savings Annualized Net Costs 1.20 0.61 2.06 II. Table of Abbreviations and Acronyms Commonly Used in This Document
Abbreviation What it means ADME Absorption, distribution, metabolism, and excretion. ANPR Advance notice of proposed rulemaking. APA Administrative Procedure Act. ASTM American Society for Testing and Materials International. ATCC American Type Culture Collection. ATE Average Treatment Effect. CDC Centers for Disease Control and Prevention. CFR Code of Federal Regulations. Start Printed Page 60477 DART Developmental and reproductive toxicity. FDA Food and Drug Administration. FD&C Act Federal Food, Drug, and Cosmetic Act. FR Federal Register. GRAE Generally recognized as effective. GRAS Generally recognized as safe. ICH International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. MBC Minimum bactericidal concentration. MIC Minimum inhibitory concentration. MusT Maximal usage trial. NCE New chemical entity. NDA New drug application. NDAC Nonprescription Drugs Advisory Committee. NHS Nurses' Health Study. NIH National Institutes of Health. NOAEL No observed adverse effect level. OMB Office of Management and Budget. OTC Over-the-counter. PBPK Physiologically-based pharmacokinetic. PK Pharmacokinetic. PR Proposed rule. TFM Tentative final monograph. U.S.C. United States Code. USP United States Pharmacopeia. III. Introduction
In the following sections, we provide a brief description of terminology used in the OTC Drug Review regulations, an overview of OTC topical antiseptic drug products, and a more detailed description of the OTC health care antiseptic active ingredients that are the subject of this final rule.
A. Terminology Used in the OTC Drug Review Regulations
1. Proposed, Tentative Final, and Final Monographs
To conform to terminology used in the OTC Drug Review regulations (§ 330.10 (21 CFR 330.10)), the advance notice of proposed rulemaking (ANPR) that was published in the Federal Register of September 13, 1974 (39 FR 33103) (the 1974 ANPR), was designated as a “proposed monograph.” Similarly, the notices of proposed rulemaking, which were published in the Federal Register of January 6, 1978 (43 FR 1210) (the 1978 TFM); the Federal Register of June 17, 1994 (59 FR 31402) (the 1994 TFM); and the Federal Register of May 1, 2015 (80 FR 25166) (the 2015 Health Care Antiseptic PR), were each designated as a TFM (see table 1 in section IV.A).
2. Category I, II, and III Classifications
The OTC drug regulations in § 330.10 use the terms “Category I” (generally recognized as safe and effective and not misbranded), “Category II” (not generally recognized as safe and effective or misbranded), and “Category III” (available data are insufficient to classify as safe and effective, and further testing is required). Section 330.10 provides that any testing necessary to resolve the safety or effectiveness issues that resulted in an initial Category III classification, and submission to FDA of the results of that testing or any other data, must be done during the OTC drug rulemaking process before the establishment of a final monograph (i.e., a final rule or regulation). Therefore, the proposed rules (at the tentative final monograph stage) used the concepts of Categories I, II, and III.
At this final monograph stage, FDA does not use the terms “Category I,” “Category II,” and “Category III.” Instead, the term “monograph conditions” is used in place of Category I, and “nonmonograph conditions” is used in place of Categories II and III.
B. Topical Antiseptics
The OTC topical antimicrobial rulemaking has had a broad scope, encompassing drug products that may contain the same active ingredients, but that are labeled and marketed for different intended uses. The 1974 ANPR for topical antimicrobial products encompassed products for both health care and consumer use (39 FR 33103). The 1974 ANPR covered seven different intended uses for these products: (1) Antimicrobial soap; (2) health care personnel hand wash; (3) patient preoperative skin preparation; (4) skin antiseptic; (5) skin wound cleanser; (6) skin wound protectant; and (7) surgical hand scrub (39 FR 33103 at 33140). FDA subsequently identified skin antiseptics, skin wound cleansers, and skin wound protectants as antiseptics used primarily by consumers for first aid use and referred to them collectively as “first aid antiseptics.” We published a separate TFM covering first aid antiseptics in the Federal Register of July 22, 1991 (56 FR 33644). We do not discuss first aid antiseptics further in this document, and this final rule does not have an impact on the status of first aid antiseptics.
The four remaining categories of topical antimicrobials were addressed in the 1994 TFM (59 FR 31402). The 1994 TFM covered: (1) Antiseptic hand wash (i.e., consumer hand wash); (2) health care personnel hand wash; (3) patient preoperative skin preparation; and (4) surgical hand scrub (59 FR 31402 at 31442). In the 1994 TFM, FDA also identified a new category of antiseptics for use by the food industry and requested relevant data and information (59 FR 31402 at 31440). In section V.B.5, we address comments filed in this rulemaking on antiseptics for use by the food industry, but we do not otherwise discuss these antiseptics in this document. This final rule does not have an impact on the status of antiseptics for food industry use.
The 1994 TFM did not distinguish between consumer antiseptic washes and rubs and health care antiseptic washes and rubs. In the 2013 Consumer Wash PR, we proposed that our evaluation of OTC antiseptic drug products be further subdivided into health care antiseptics and consumer antiseptics (78 FR 76444 at 76446). These categories are distinct based on the proposed use setting, target population, and the fact that each Start Printed Page 60478setting presents a different level of risk for infection. In the 2013 Consumer Wash PR (78 FR 76444 at 76446 to 76447) and the 2016 Consumer Rub PR (81 FR 42912 at 42915 to 42916), we proposed that our evaluation of OTC consumer antiseptic drug products be further subdivided into consumer washes (products that are rinsed off with water, including hand washes and body washes) and consumer rubs (products that are not rinsed off after use, including hand rubs and antibacterial wipes). This final rule does not have an impact on the status of consumer antiseptic wash or consumer antiseptic rub products.
C. This Final Rule Covers Only Health Care Antiseptics
We refer to the group of products covered by this final rule as “health care antiseptics.” Health care antiseptics are drug products that are generally intended for use by health care professionals in a hospital setting or other health care situations outside the hospital. Patient antiseptic skin preparations, which are products that are used for preparation of the skin prior to surgery (i.e., preoperative) and preparation of skin prior to an injection (i.e., preinjection), may be used by patients outside the traditional health care setting. Some patients (e.g., diabetics who manage their disease with insulin injections) self-inject medications that have been prescribed by a health care professional for use at home or at other locations and use patient preoperative skin preparations prior to injection.
In this final rule, we use the term “health care antiseptics” to include the following products:
- Health care personnel hand washes
- Health care personnel hand rubs
- Surgical hand scrubs
- Surgical hand rubs
- Patient antiseptic skin preparations (i.e., patient preoperative and preinjection skin preparations) [1]
This final rule covers health care antiseptic products that are rubs and others that are washes. The 1994 TFM did not distinguish between products that we are now calling health care “antiseptic washes” and products we are now calling health care “antiseptic rubs.” Washes are rinsed off with water, and include health care personnel hand washes and surgical hand scrubs. Rubs are sometimes referred to as “leave-on products” and are not rinsed off after use. Rubs include health care personnel hand rubs, surgical hand rubs, and patient antiseptic skin preparations.
Completion of the monograph for health care antiseptic products and certain other monographs for the active ingredient triclosan is subject to a Consent Decree entered by the U.S. District Court for the Southern District of New York on November 21, 2013, in Natural Resources Defense Council, Inc. v. United States Food and Drug Administration, et al., 10 Civ. 5690 (S.D.N.Y.).
IV. Background
In this section, we describe the significant rulemakings and public meetings relevant to this rulemaking and discuss our response to comments received on the 2015 Health Care Antiseptic PR.
A. Significant Rulemakings Relevant to This Final Rule
A summary of the significant Federal Register publications relevant to this final rule is provided in table 1. Other publications relevant to this final rule are available at https://www.regulations.gov in FDA Docket No. 1975-N-0012 (formerly Docket No. 1975-N-0183H).
Table 1—Significant Rulemaking Publications Related to Health Care Antiseptic Drug Products 1
Federal Register notice Information in notice 1974 ANPR (September 13, 1974, 39 FR 33103) We published an ANPR to establish a monograph for OTC topical antimicrobial drug products, together with the recommendations of the advisory review panel (the Panel) responsible for evaluating data on the active ingredients in this drug class. 1978 Antimicrobial TFM (January 6, 1978, 43 FR 1210) We published our tentative conclusions and proposed effectiveness testing for the drug product categories evaluated by the Panel, reflecting our evaluation of the Panel's recommendations and comments and data submitted in response to the Panel's recommendations. 1991 First Aid TFM (July 22, 1991, 56 FR 33644) We amended the 1978 TFM to establish a separate monograph for OTC first aid antiseptic products. In the 1991 TFM, we proposed that first aid antiseptic drug products be indicated for the prevention of skin infections in minor cuts, scrapes, and burns. 1994 Healthcare Antiseptic TFM (June 17, 1994, 59 FR 31402) We amended the 1978 TFM to establish a separate monograph for the group of products referred to as OTC topical health care antiseptic drug products. These antiseptics are generally intended for use by health care professionals. In the 1994 TFM, we also recognized the need for antibacterial personal cleansing products for consumers to help prevent cross-contamination from one person to another and proposed a new antiseptic category for consumer use: Antiseptic hand wash. 2013 Consumer Antiseptic Wash TFM (December 17, 2013, 78 FR 76444) We issued a proposed rule to amend the 1994 TFM and to establish data standards for determining whether OTC consumer antiseptic washes are GRAS/GRAE. In the 2013 Consumer Antiseptic Wash TFM, we proposed that additional safety and effectiveness data are necessary to support the safety and effectiveness of consumer antiseptic wash active ingredients. 2015 Health Care Antiseptic TFM (May 1, 2015, 80 FR 25166) We issued a proposed rule to amend the 1994 TFM and to establish data standards for determining whether OTC health care antiseptics are GRAS/GRAE. In the 2015 Health Care Antiseptic TFM, we proposed that additional data are necessary to support the safety and effectiveness of health care antiseptic active ingredients. 2016 Consumer Antiseptic Rub TFM (June 30, 2016, 81 FR 42912) We issued a proposed rule to amend the 1994 TFM and to establish data standards for determining whether OTC consumer antiseptic rubs are GRAS/GRAE. In the 2016 Consumer Antiseptic Rub TFM, we proposed that additional safety and effectiveness data are necessary to support the safety and effectiveness of consumer antiseptic rub active ingredients. Start Printed Page 60479 2016 Consumer Antiseptic Wash Final Monograph (September 6, 2016, 81 FR 61106) We issued a final rule finding that certain active ingredients used in OTC consumer antiseptic wash products are not GRAS/GRAE. We deferred further rulemaking on three specific active ingredients (benzalkonium chloride, benzethonium chloride, and chloroxylenol) used in OTC consumer antiseptic wash products to allow for the development and submission of new safety and effectiveness data to the record for those ingredients. 1 The publications listed in table 1 can be found at FDA's “Status of OTC Rulemakings” website available at http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Over-the-CounterOTCDrugs/StatusofOTCRulemakings/ucm070821.htm. The publications dated after 1993 can also be found in the Federal Register at https://www.federalregister.gov. B. Public Meetings Relevant to This Final Rule
In addition to the Federal Register publications listed in table 1, there have been three meetings of the NDAC that are relevant to the discussion of health care antiseptic safety and effectiveness. These meetings are summarized in table 2.
Table 2—Public Meetings Relevant to Health Care Antiseptics
Date and type of meeting Topic of discussion January 1997, NDAC Meeting (Joint meeting with the Anti-Infective Drugs Advisory Committee) (January 6, 1997, 62 FR 764) Antiseptic and antibiotic resistance in relation to an industry proposal for consumer and health care antiseptic effectiveness testing (Health Care Continuum Model) (Refs. 1 and 2). March 2005, NDAC Meeting (February 18, 2005, 70 FR 8376) The use of surrogate endpoints and study design issues for the in vivo testing of health care antiseptics (Ref. 3). September 2014, NDAC Meeting (July 29, 2014, 79 FR 44042) Safety testing framework for health care antiseptic active ingredients (Ref. 4). C. Scope of This Final Rule
This rulemaking finalizes the nonmonograph status of the 24 listed health care antiseptic active ingredients (see section IV.D.1). Requests were made that benzalkonium chloride, benzethonium chloride, chloroxylenol, alcohol, isopropyl alcohol, and povidone-iodine be deferred from consideration in this health care antiseptic final rule to allow more time for interested parties to complete the studies necessary to fill the safety and effectiveness data gaps identified in the 2015 Health Care Antiseptic PR for these ingredients. In January 2017, we agreed to defer rulemaking on these six ingredients (see Docket No. 2015-N-0101 at https://www.regulations.gov).
For the 24 active ingredients included in this final rule, no additional data were submitted to the record to fill the safety and effectiveness data gaps identified in the 2015 Health Care Antiseptic PR for these 24 active ingredients. Therefore, we find that these 24 active ingredients are not GRAS/GRAE for use in health care antiseptic drug products and these ingredients are not included in the OTC topical antiseptic monograph at this time. Products containing these ingredients are new drugs for which approved new drug applications (NDAs) or abbreviated new drug applications (ANDAs) are required prior to marketing. Accordingly, FDA is amending part 310 (21 CFR part 310) to add the active ingredients covered by this final rule to the list of active ingredients in § 310.545 (21 CFR 310.545) that are not GRAS/GRAE for use in the specified OTC drug products.
D. Eligibility for the OTC Drug Review
An OTC drug is covered by the OTC Drug Review if its conditions of use existed in the OTC drug marketplace on or before May 11, 1972 (37 FR 9464) (Ref. 5).[2] Conditions of use include, among other things, active ingredient, dosage form and strength, route of administration, and specific OTC use or indication of the product (see § 330.14(a)). To determine eligibility for the OTC Drug Review, FDA typically must have actual product labeling or a facsimile of labeling that documents the conditions of marketing of a product before May 1972 (see § 330.10(a)(2)). FDA considers a drug that is ineligible for inclusion in the OTC monograph system to be a new drug that requires FDA approval of an NDA or ANDA. Ineligibility for use as a health care antiseptic does not affect eligibility under any other OTC drug monograph.
1. Eligible Active Ingredients
Table 3 lists the health care antiseptic active ingredients that have been considered under this rulemaking and shows whether each ingredient is eligible or ineligible for evaluation under the OTC Drug Review for use in health care antiseptics for each of the five specified uses: Patient antiseptic skin preparation, health care personnel hand wash, health care personnel hand rub, surgical hand scrub, and surgical hand rub.Start Printed Page 60480
Table 3—Eligibility of Antiseptic Active Ingredients for Health Care Antiseptic Uses 1
Active ingredient Patient antiseptic skin preparation Health care personnel hand wash Health care personnel hand rub Surgical hand scrub Surgical hand rub Alcohol 60 to 95 percent 2 Y 3 N Y N Y Benzalkonium chloride Y Y Y Y N Benzethonium chloride Y Y N Y N Chlorhexidine gluconate N N N N N Chloroxylenol Y Y N Y N Cloflucarban Y Y N Y N Fluorosalan Y Y N Y N Hexylresorcinol Y Y N Y N Iodine complex (ammonium ether sulfate and polyoxyethylene sorbitan monolaurate) N Y N Y N Iodine complex (phosphate ester of alkylaryloxy polyethylene glycol) Y Y N Y N Iodine tincture United States Pharmacopeia (USP) Y N N N N Iodine topical solution USP Y N N N N Nonylphenoxypoly (ethyleneoxy) ethanoliodine Y Y N Y N Poloxamer-iodine complex Y Y N Y N Povidone-iodine 5 to 10 percent Y Y N Y N Undecoylium chloride iodine complex Y Y N Y N Isopropyl alcohol 70-91.3 percent Y N Y N Y Mercufenol chloride Y N N N N Methylbenzethonium chloride Y Y N Y N Phenol (equal to or less than 1.5 percent) Y Y N Y N Phenol (greater than 1.5 percent) Y Y N Y N Secondary amyltricresols Y Y N Y N Sodium oxychlorosene Y Y N Y N Triclocarban Y Y N Y N Triclosan Y Y N Y N Combinations: Calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative Y N N N N Mercufenol chloride and secondary amyltricresols in 50 percent alcohol Y N N N N Triple dye Y N N N N 1 Hexachlorophene and tribromsalan are not included in this table because they are the subject of final regulatory action (see section IV.D.3). 2 Y = Eligible for specified use. 3 N = Ineligible for specified use. 2. Ineligible Active Ingredients
In the 2015 Health Care Antiseptic PR (and as outlined in table 3), we identified certain active ingredients that were considered ineligible for evaluation under the OTC Drug Review as a health care antiseptic for specific indications. We noted, however, that if the requested documentation for eligibility was submitted, these active ingredients could be determined to be eligible for evaluation (80 FR 25166 at 25171).
We received a comment requesting that benzethonium chloride be deemed eligible for evaluation under the OTC Drug Review for use as a health care personnel hand rub and surgical hand rub. For the reasons explained in section V.C.1, we find that benzethonium chloride continues to be ineligible for evaluation under the OTC Drug Review for use as a health care personnel hand rub and surgical hand rub. Consequently, drug products containing benzethonium chloride for use in health care personnel hand rubs and surgical hand rubs will require approval under an NDA or ANDA prior to marketing.
We also received comments arguing that chlorhexidine gluconate is eligible for evaluation under the OTC Drug Review for use as a health care antiseptic. For the reasons explained in section V.C.2, we find that chlorhexidine gluconate continues to be ineligible for evaluation under the OTC Drug Review for use as a health care antiseptic. Consequently, drug products containing chlorhexidine gluconate for use in health care antiseptics will require approval under an NDA or ANDA prior to marketing.
In addition, we received a comment requesting that alcohol be deemed eligible for evaluation under the OTC Drug Review for use as a surgical hand scrub. For the reasons explained in section V.C.3, we find that alcohol continues to be ineligible for evaluation under the OTC Drug Review for use as a surgical hand scrub. Consequently, drug products containing alcohol for use in surgical hand scrubs will require approval under an NDA or ANDA prior to marketing.
Moreover, for the remaining health care antiseptic active ingredients that we proposed were ineligible for evaluation under the OTC Drug Review, we have not received any new information since the publication of the 2015 Health Care Antiseptic PR demonstrating that these ineligible active ingredients are eligible for Start Printed Page 60481evaluation under the OTC Drug Review for use as a health care antiseptic for the specified indications (see table 3). Consequently, we find that these active ingredients continue to be ineligible for evaluation under the OTC Drug Review for use as a health care antiseptic for the specified indications and drug products containing these ineligible active ingredients will require approval under an NDA or ANDA prior to marketing.
3. Ingredients Previously Proposed as Not Generally Recognized as Safe and Effective
FDA may determine that an active ingredient is not GRAS/GRAE for a given OTC use (i.e., nonmonograph) because of lack of evidence of effectiveness, lack of evidence of safety, or both. In the 1994 TFM (59 FR 31402 at 31435 to 31436) and the 2015 Health Care Antiseptic PR (80 FR 25166 at 25173 to 25174), FDA proposed that the active ingredients fluorosalan, hexachlorophene, phenol (greater than 1.5 percent), and tribromsalan be found not GRAS/GRAE for the uses set forth in the 1994 TFM: Antiseptic hand wash, health care personnel hand wash, patient antiseptic skin preparation, and surgical hand scrub. FDA did not classify hexachlorophene or tribromsalan in the 1978 TFM (43 FR 1210 at 1227) because it had already taken final regulatory action against hexachlorophene (21 CFR 250.250) and certain halogenated salicylamides, notably tribromsalan (21 CFR 310.502). No substantive comments or new data were submitted to the record of the 1994 TFM or the 2015 Health Care Antiseptic PR to support reclassification of any of these ingredients as GRAS/GRAE. Therefore, FDA has determined that these active ingredients are not GRAS/GRAE for use in OTC health care antiseptic products as defined in this final rule, and drug products containing these ineligible active ingredients will require approval under an NDA or ANDA prior to marketing.
V. Comments on the Proposed Rule and FDA Response
A. Introduction
In response to the 2015 Health Care Antiseptic PR, we received approximately 29 comments from drug manufacturers, trade associations, academia, testing laboratories, health professionals, and individuals. We also received additional data and information for certain deferred health care antiseptic active ingredients.
We describe and respond to the comments in section V.B through V.F. We have numbered each comment to help distinguish among the different comments. We have grouped similar comments together under the same number, and in some cases, we have separated different issues discussed in the same comment and designated them as distinct comments for purposes of our responses. The number assigned to each comment or comment topic is purely for organizational purposes and does not signify the comment's value, importance, or the order in which comments were received.
B. General Comments on the Proposed Rule and FDA Response
1. Effective Date
(Comment 1) Several comments requested that FDA extend its timeline under the 2015 Health Care Antiseptic PR to allow more time for the submission of new data and information. They asserted that the one year compliance date was too short and that it could take several years to design, execute, analyze, and report on the necessary safety and effectiveness studies.
(Response 1) In the 2015 Health Care Antiseptic PR, we provided a process for seeking an extension of time to submit the required safety and effectiveness data if such an extension is necessary (80 FR 25166 at 25169). As explained in the proposed rule, we stated that we would consider all the data and information submitted to the record in conjunction with all timely and completed requests to extend the timeline to finalize the monograph status for a given ingredient. We received requests to defer six health care antiseptic active ingredients from this rulemaking. Consideration for deferral for an ingredient was given to requests with clear statements of intent to conduct the necessary studies required to fill all the data gaps identified in the proposed rule for that ingredient. After analyzing the data and information submitted related to the requests for extensions, we determined that a deferral is warranted for the six health care antiseptic active ingredients—benzalkonium chloride, benzethonium chloride, chloroxylenol, alcohol, isopropyl alcohol, and povidone-iodine—to allow more time for interested parties to complete the studies necessary to fill the safety and effectiveness data gaps identified for these ingredients in the 2015 Health Care Antiseptic PR. The monograph status of these six ingredients will be addressed either after completion and analysis of ongoing studies to address the safety and effectiveness data gaps of these ingredients or at a later date if these studies are not completed. We did not receive any deferral requests for the 24 remaining health care antiseptic active ingredients, and so we decline to defer final action on the proposed rule for these ingredients.
2. Use in Health Care Settings Outside the Hospital
(Comment 2) One comment requested that FDA “better clarify and define the scope” of this rulemaking on the use of health care antiseptics in health care settings outside of the hospital “in order that the proper antiseptic products are provided for patients in the spectrum of health care settings while also being covered by health care insurers.” The comment stated that patients and health care workers in these other settings deserve the same level of safety and efficacy standards as those in the hospital setting. The comment expressed concern that certain entities may determine that they need to supply products intended for “consumer use,” which, the comment stated, may have different and lesser standards.
(Response 2) We agree that health care antiseptic products are used in a variety of health care settings, not just hospitals. Over the past several decades, there has been a significant shift in health care delivery from the acute, inpatient hospital setting to a variety of outpatient and community-based settings. There are many examples of health care settings outside the hospital that involve the use of antiseptic products. These settings include, but are not limited to, the care of patients in outpatient medical and surgical facilities, dental clinics, skilled nursing facilities or nursing homes, adult medical day care centers, public health clinics, imaging centers, oncology clinics, infusion centers, dialysis centers, behavioral health clinics, physical therapy and rehabilitation centers, and in private homes. The term “health care” as used in this rulemaking includes all these settings.
We note, however, that this rule does not address the use of a specific health care antiseptic drug product in a particular health care situation. In addition, the coverage of antiseptic drug products by health care insurers is outside FDA's purview.
3. GRAS/GRAE Classification of Certain Ingredients
(Comment 3) Several comments requested that FDA reconsider its proposal in the 2015 Health Care Antiseptic PR to classify alcohol, isopropyl alcohol, and povidone-iodine as Category III active ingredients. In the 1994 TFM, alcohol, isopropyl alcohol, Start Printed Page 60482and povidone-iodine were proposed to be classified as Category I topical antiseptic ingredients for certain indications. The comments contended that FDA's proposal to change these ingredients' proposed classification from Category I to Category III is not based on a safety or effectiveness concern or issue. One comment noted that during the September 3, 2014, NDAC meeting, several NDAC members expressed concerns about changing the proposed classification of alcohol, isopropyl alcohol, and povidone-iodine from Category I to Category III, indicating that the change in the proposed classification could lead health care personnel to stop using products with these active ingredients. The comment also pointed out that, in the 2015 Health Care Antiseptic PR and in related public announcements, FDA emphasized that we did not believe that health care antiseptic products containing these ingredients were ineffective or unsafe, or that their use should be discontinued. In fact, that comment noted that FDA recommended that health care personnel continue to use these antiseptic products consistent with infection control guidelines while additional data about the products were gathered.
(Response 3) As we explained in the 2015 Heath Care Antiseptic PR, the OTC drug procedural regulations in § 330.10 use the terms “Category I” (generally recognized as safe and effective and not misbranded), “Category II” (not generally recognized as safe and effective or misbranded), and “Category III” (available data are insufficient to classify as safe and effective, and further testing is required) (80 FR 25166 at 25168). We classify ingredients as Category I, II, or III until the final monograph stage, at which point we use the term “monograph conditions” in place of Category I, and the term “nonmonograph conditions” in place of Categories II and III. In the 1994 TFM, alcohol and povidone-iodine were both proposed to be classified as Category I topical antiseptic ingredients for use in surgical hand scrubs, patient antiseptic skin preparations, and antiseptic hand washes or health care personnel hand wash products (59 FR 31402 at 31420 and 31433). Isopropyl alcohol was proposed to be classified as Category I for patient antiseptic skin preparation “for the preparation of the skin prior to an injection” (59 FR 31402 at 31433).
In the 2015 Health Care Antiseptic PR, we changed the proposed classification of alcohol, isopropyl alcohol, and povidone-iodine from Category I to III for these indications, because we found that there was not enough data on these three ingredients to meet our proposed safety and effectiveness data requirements. We explained that we were proposing changes to the safety and effectiveness data requirements identified in the 1994 TFM in light of comments we received, input from subsequent public meetings, and our independent evaluation of other relevant scientific information (80 FR 25166 at 25166).
Among other things, our proposed revisions to the data requirements identified in the 1994 TFM were based on several important scientific developments that affected the safety evaluation of health care antiseptic active ingredients, including improved analytical methods that can detect and more accurately measure these ingredients at lower levels in the bloodstream and tissue (80 FR 25166 at 25166 to 25167). As a result of these improved methods, we have learned that some systemic exposures can be detected, where previously they were undetected, and that some systemic exposures are higher than previously thought. We also have new information about the potential risks from systemic absorption and long-term exposure (80 FR 25166 at 25167). In addition, the standard battery of tests that were used to determine the safety of drugs had changed over time to incorporate improvements in safety testing. As we explained in the 2015 Health Care Antiseptic PR, it is critical that the safety and effectiveness of these ingredients be supported by data that meet the most current standards, considering the prevalent use of health care antiseptic products (80 FR 25166 at 25167).
Our decision to propose revising the safety and effectiveness data requirements identified in the 1994 TFM was also based in part on meetings of the NDAC that were held in March 2005 and September 2014. As we noted in the preamble to the 2015 Health Care Antiseptic PR, input from participants at the March 2005 NDAC meeting prompted us to reevaluate the data needed for classifying health care antiseptic active ingredients as GRAE (80 FR 25166 at 25166). Moreover, at the meeting held in September 2014, the NDAC discussed FDA's proposed revisions to the safety data requirements and unanimously voted that the revised safety data requirements were appropriate to demonstrate that a health care antiseptic active ingredient is GRAS.
As one comment noted, at the September 2014 meeting, several NDAC members expressed concerns about changing the proposed classification of alcohol, isopropyl alcohol, and povidone-iodine from Category I to Category III, indicating that this change in the proposed classification could lead health care personnel to stop using products with these active ingredients. At the same meeting, FDA emphasized both that health care antiseptics are a critically important part of the infection control paradigm in place in every hospital across the country and that our goal is not to remove such products from the market (Ref. 4). That remains our goal, and we note that these ingredients have each been deferred, so they are not addressed in this final rule.
4. Patient Preoperative Skin Preparation
(Comment 4) One comment asked FDA to clarify the term “patient preoperative skin preparation,” noting that, in the 2015 Health Care Antiseptic PR, the term “patient preoperative skin preparation” includes skin preparation prior to an injection (preinjection) and that this may cause confusion because it could be misinterpreted to mean that all products listed can be used for either patient preoperative skin preparation or preinjection.
Several comments also asserted that the effectiveness testing for preinjection should have different clinically relevant time points because preinjection use serves a different purpose and has a different use pattern than patient preoperative skin preparations. They argued that surgical incision demands persistent activity due to the invasive nature of cutting through the skin's natural barrier over a larger area, the procedure duration (which can be hours), and the time the incision point will be open and will subsequently need to heal. As such, the comments argued, persistence may be an important attribute of patient preoperative skin preparations. They explained that in contrast, an injection is a procedure lasting only seconds and poses a relatively low risk of infection. They also explained that the injection site heals quickly, so there is no need for persistent antimicrobial activity. They stated that if patient preinjection skin preparation products are required to meet the same effectiveness requirements as patient preoperative skin preparation products, this would effectively clear the market of available cost effective solutions for those who need these products. Therefore, the comments asserted that the effectiveness requirements for patient preoperative skin preparation should be different from the effectiveness requirements for patient preinjection skin preparations.
(Response 4) We agree that the circumstances under which health care Start Printed Page 60483antiseptics can be used for preinjection should be clarified because patient preoperative skin preparations and preinjection skin preparations can serve different purposes and have different uses. Accordingly, we clarify that patient preoperative skin preparation and patient preinjection skin preparation may involve separate uses within the category of patient antiseptic skin preparations. As noted in the comments, surgical incisions require persistent activity from patient preoperative skin preparations due to the invasive nature of cutting through the skin's natural barrier over a larger area, the procedure duration (which can be hours), and the time the incision point will be open and will subsequently need to heal. As such, persistence is an important attribute of patient preoperative skin preparations. In comparison, injection refers to a brief interruption of skin integrity by a sterile needle that is typically removed within seconds or a few minutes. Due to the brevity of the procedure, the risk of bacterial infection from an injection is low, and so persistent antimicrobial activity is not essential for a preinjection skin preparation product.
Examples of procedures that are covered by a preinjection claim include the following:
- Intramuscular injection for vaccination
- Intramuscular injection for delivery of medication, such as an antibiotic or an anesthetic (for trigger point injection)
- Intradermal injection for tuberculin testing
- Subcutaneous injection of insulin
- Subcutaneous placement of needles for acupuncture
- Venipuncture for blood drawing for laboratory testing
- Intradermal injection for allergy skin testing
Examples of procedures that are not covered by the preinjection claim include the following:
- Venous catheterization for blood donation
- Venous catheterization for an extended delivery of medication, such as slow infusion of an antibiotic
- Venous catheterization for delivery of intravenous fluid
- Placement of a central venous catheter for any purpose
- Placement of a heparin lock
- Placement of an arterial catheter
- Surgical procedure
As stated in the 2015 Health Care Antiseptic PR (80 FR 25166 at 25176), the effectiveness criteria for health care antiseptics are based on the premise that bacterial reductions achieved using tests that simulate conditions of actual use for each OTC health care antiseptic product reflect the bacterial reductions that would be achieved under conditions of such use. Thus, the effectiveness requirements for determining whether an active ingredient is GRAE for use in patient preinjection skin preparations should be consistent with the actual use of that product. We agree that patient antiseptic skin preparations used for preinjection involve a process lasting a much shorter period of time, sometime seconds, compared to surgery, which can last several hours, and that such preinjection use has a lower risk of infection. For these reasons, we also agree that the effectiveness requirements for preinjection should be different than the effectiveness requirements for patient preoperative skin preparations. We discuss these effectiveness requirements in more detail in section V.D.2.
We also note that, although we do not address labeling in this final rule because at this time we have not found any active ingredients to be GRAS/GRAE for use in patient antiseptic skin preparations, we anticipate that labeling for these products will include directions for use that will help providers determine the proper use of preoperative and preinjection antiseptic products.
5. Food Handler Antiseptics
(Comment 5) Several comments requested that FDA formally recognize antiseptic hand washes and rubs used in the food industry as a distinct food handler category subject to its own monograph. The comments also requested that FDA confirm that food handler antiseptics can continue to be marketed until FDA issues a food handler monograph.
(Response 5) As stated in the 2016 Consumer Wash Final Rule (81 FR 61106 at 61109) and the 2015 Health Care Antiseptic PR (80 FR 25166 at 25168), we continue to classify the food handler antiseptic washes as a separate and distinct monograph category. As explained in those rulemakings, food handler antiseptic products are not part of these rulemakings on the health care and consumer antiseptic monographs. We continue to believe a separate category is warranted because of additional issues raised by the public health consequences of foodborne illness, differences in frequency and type of use, and contamination of the hands by grease and other oils.
C. Comments on Eligibility of Active Ingredients and FDA Response
1. Benzethonium Chloride
(Comment 6) In response to the 2015 Health Care Antiseptic PR, we received a comment asserting that benzethonium chloride is eligible for review under the monograph for use in health care personnel hand rubs and surgical hand rubs and that benzethonium chloride be categorized as a Category I ingredient for both indications. Information submitted in the comment showed that methylbenzethonium chloride was present in Bactine, a topical antiseptic for first aid and wound care before May 1972. The comment also asserted that:
- Methylbenzethonium chloride was the active ingredient in the antiseptic, Bactine.
- Bactine with methylbenzethonium chloride was in use before 1972 as a leave-on antiseptic (not rinsed off).
- Methylbenzethonium chloride and benzethonium chloride are equivalent.
- The conditions of use for benzethonium chloride in the 2015 Health Care Antiseptic PR are the same as for Bactine.
(Response 6) In the 2015 Health Care Antiseptic PR (80 FR 25166 at 25171), we explained that an OTC drug is covered by the OTC Drug Review if its conditions of use existed in the OTC drug marketplace on or before May 11, 1972. Conditions of use include active ingredient, dosage form and dosage strength, route of administration, and the specific OTC use or indication of the product. If the eligibility of a product for OTC Drug Review is in question, FDA must have actual product labeling or a facsimile of labeling that documents the conditions of marketing the product before May 1972 (see § 330.10(a)(2)). If benzethonium chloride was the active ingredient in a drug before May 1972 for use as a health care personnel hand rub and/or surgical hand rub, then it would be eligible for the OTC Drug Review for those indications.
We disagree with the comment's statement asserting that methylbenzethonium chloride (the active ingredient in Bactine) is essentially equivalent to benzethonium chloride based on their similar structure and chemical function (both are quaternary ammonium chloride antiseptic ingredients). Although these two ingredients are chemically similar such that they could be grouped as quaternary ammonium compounds, they are not equivalent molecules. Furthermore, although not suggested by the comment, there is no evidence that methylbenzethonium is a prodrug for benzethonium chloride, or requires Start Printed Page 60484conversion or metabolism to benzethonium chloride for antiseptic activity when applied to the skin.
Moreover, although the comment provided data to demonstrate that methylbenzethonium chloride was used in Bactine before May 1972, the submitted label for Bactine contained indications that are not equivalent to the indications for health care personnel hand rubs or surgical hand rubs. The indications and directions on the Bactine label (i.e., minor cuts, scratches, and abrasions; minor burns, sunburn; itching skin irritations; shaving antiseptic; sickroom, nursery (hands, thermometers, surgical instruments, sickroom articles); athlete's foot—sore tired feet) do not support the use of benzethonium chloride as an active ingredient used in a health care antiseptic hand rub by a health care professional in the care of patients or by a surgeon before surgery. The Directions for Use (indications) from the Bactine bottle do not support the eligibility of methylbenzethonium chloride as an OTC health care antiseptic hand rub or surgical hand rub. Lastly, although the use of methylbenzethonium chloride to disinfect the hands is suggested by the word “hands” in the directions for “sickroom, nursery (hands, thermometers, surgical instruments, sickroom articles) use full strength Bactine,” this reference to hands is imprecise and no specific Directions for Use are provided.
We also performed a literature search to investigate whether benzethonium chloride was used as an active ingredient in an OTC health care antiseptic leave-on product for the indication of a health care personnel hand rub or surgical hand rub before May 1972. Our search did not find evidence for the use of benzethonium chloride as a health care personnel hand rub or surgical hand rub.
In sum, we find that the data submitted in support of the eligibility of benzethonium chloride as a monograph active ingredient for use as a health care personnel hand rub and/or a surgical hand rub do not demonstrate that benzethonium chloride is eligible for use for these health care antiseptic indications. For these reasons, we find that benzethonium chloride continues to be ineligible for evaluation under the OTC Drug Review for use as a health care personnel hand rub and surgical hand rub. Consequently, drug products containing benzethonium chloride for use in health care personnel hand rubs and surgical hand rubs will require approval under an NDA or ANDA prior to marketing.
2. Chlorhexidine Gluconate
(Comment 7) FDA received two comments asserting that chlorhexidine gluconate should be eligible for inclusion in the OTC health care antiseptic monograph. The comments also stated that more data are needed to find chlorhexidine gluconate GRAS/GRAE for use as an OTC health care antiseptic.
(Response 7) Chlorhexidine gluconate was not included in the 1994 TFM because we had previously found chlorhexidine gluconate to be ineligible for inclusion in the monograph for any health care antiseptic use (80 FR 25166 at 25172, citing 59 FR 31402 at 31413). In the 2015 Health Care Antiseptic PR, we explained that we had not received any new information since the 1994 TFM that supported the eligibility of chlorhexidine gluconate for inclusion in the monograph. Consequently, we proposed not to change the categorization of chlorhexidine gluconate based on the lack of documentation demonstrating its eligibility under the OTC Drug Review for use as a health care antiseptic (80 FR 25166 at 25172).
The comments on chlorhexidine gluconate submitted in response to the 2015 Health Care Antiseptic PR did not include any data or any new information to support chlorhexidine gluconate's eligibility for inclusion in the health care antiseptic monograph. Specifically, no evidence was submitted for chlorhexidine gluconate to demonstrate that chlorhexidine gluconate was an active ingredient in OTC health care antiseptics in the United States before May 1972. Consequently, we find that chlorhexidine gluconate continues to be ineligible for evaluation under the OTC Drug Review for use as a health care antiseptic. Drug products containing chlorhexidine gluconate for use in health care antiseptics will require approval under an NDA or ANDA prior to marketing. Because chlorhexidine gluconate continues to be ineligible for consideration under the health care antiseptic monograph, it is unnecessary to address the comments' statement that more safety and effectiveness data are needed to find chlorhexidine gluconate GRAS/GRAE for OTC health care antiseptic use.
(Comment 8) In response to the 2015 Health Care Antiseptic PR, we also received a comment expressing concerns regarding the bacterial resistance of chlorhexidine gluconate. In addition, we received a comment that suggested that chlorhexidine gluconate is superior to povidone-iodine as a patient preoperative skin preparation.
(Response 8) Because we find that chlorhexidine gluconate is ineligible for consideration under the health care antiseptic monograph and these comments do not have an impact on this finding, we do not address these comments in this final rule.
3. Alcohol
(Comment 9) In response to the 2015 Health Care Antiseptic PR, a comment was submitted that argued that alcohol should be deemed eligible for evaluation under the OTC Drug Review for use as a surgical hand scrub. The comment asserted that FDA first made its distinction between “rubs” and “scrubs” in the 2015 Health Care Antiseptic PR, in which FDA proposed that alcohol was ineligible for inclusion in the health care antiseptic monograph as a surgical hand scrub. The comment stated that FDA based this conclusion on the fact that information for rinse-off products was not submitted to the OTC Drug Review. But, the comment claimed, manufacturers had no reason to submit such information because FDA had found alcohol to be GRAS/GRAE for use in surgical hand scrub products in the 1994 TFM, and manufacturers had no notice that FDA was expecting such submissions. The comment argued that the Agency's exclusion of alcohol from the 2015 Health Care Antiseptic PR for use as a surgical hand scrub was arbitrary and capricious and in violation of the Administrative Procedure Act (APA), 5 U.S.C.A. sections 501 et seq.
(Response 9) In the 2015 Health Care Antiseptic PR, we explained that the 1994 TFM did not distinguish between products that we are now calling “antiseptic washes” and products we are now calling “antiseptic rubs.” However, based on comments submitted in response to the 1994 TFM, we tentatively determined that there should be a distinction between antiseptic washes and antiseptic rubs, as well as a distinction between consumer antiseptic and health care antiseptic products. As evidenced by the comments received in response to the 1994 TFM, formulation practices and marketing intent of these products has changed over time and products may not be eligible for conditions under which they are currently marketed. We explained that washes are rinsed off with water, and include health care personnel hand washes and surgical hand scrubs, while rubs are sometimes referred to as “leave-on products” and are not rinsed off after use, and include health care personnel hand rubs, Start Printed Page 60485surgical hand rubs, and patient preoperative skin preparations (80 FR 25166 at 25169). As a result of these distinctions, we proposed that alcohol was ineligible for use as a health care personnel hand wash and surgical hand scrub because the only health care antiseptic products that contained alcohol for which evidence was submitted to the OTC Drug Review for evaluation were products that were intended to be used without water (i.e., rubs and skin preparations) (Id. at 25172).
We disagree with the comment's assertions that manufacturers did not have notice or an opportunity to submit information to the OTC Drug Review on alcohol's eligibility for use as a surgical hand scrub. First, we note that the 1994 TFM was a proposed rule, not a final rule; we proposed, but had not yet found, alcohol to be GRAS/GRAE for use in surgical hand scrub products. Moreover, in the 2015 Health Care Antiseptic PR, our proposal that alcohol was ineligible for use as a surgical hand scrub also was a preliminary determination based on the lack of adequate evidence of eligibility for evaluation under the OTC Drug Review. In the proposed rule, we invited parties to submit such evidence of eligibility. We explained that if the documentation demonstrated that an active ingredient met the OTC Drug Review requirements, the active ingredient could be determined to be eligible for evaluation for the specified use. Parties had 180 days to submit comments on the proposed rule and 12 months to submit any new data or information on the proposed rule, including evidence and documentation on eligibility (80 FR 25166 at 25169). The comment submitted in response to the 2015 Health Care Antiseptic PR on this issue did not include any documentation or evidence to demonstrate that alcohol is eligible for use as a surgical hand scrub under the OTC antiseptic monograph, despite the opportunity to include such information. Also, there was no additional data or information submitted to the record thereafter to demonstrate alcohol's eligibility for evaluation under the OTC Drug Review for use as a surgical hand scrub.
For these reasons, we find that alcohol continues to be ineligible for evaluation under the OTC Drug Review for use as a surgical hand scrub. Consequently, drug products containing alcohol for use in surgical hand scrubs will require approval under an NDA or ANDA prior to marketing.
We also note that where these active ingredients are ineligible for evaluation under the OTC Drug Review, interested parties may have the option to submit a time and extent application under § 330.14 (21 CFR 330.14) of FDA's regulations to request that the Agency amend the health care antiseptic monograph to include these active ingredients for use in health care antiseptics for the specified indications.
D. Comments on Effectiveness and FDA Response
1. Clinical Simulation Studies
(Comment 10) One comment stated that FDA should require the same clinical studies that were required to show a benefit of OTC consumer antiseptic washes over and above washing with non-antibacterial soap for OTC antiseptics used in the health care setting. The comment asserted that there are numerous safety concerns with the use of these active ingredients and given these concerns and health care workers' extensive exposure to these ingredients in their workplaces on a daily basis, the Agency should find that there is a benefit over and above washing with plain soap and water in order to make a GRAE determination for these active ingredients. The comment stated that if FDA relies on bacterial reduction as a proxy for effectiveness in the health care setting, it must require that that reduction be compared against plain soap and water, especially given that workers in the health care setting likely wash their hands more frequently than the general public, and thus, are exposed to higher levels of these ingredients.
(Response 10) As we explained in the 2015 Health Care Antiseptic PR (80 FR 25166 at 25175 to 25176), study design limitations and ethical concerns prevent the use of clinical outcome studies to demonstrate the effectiveness of active ingredients used in health care antiseptic products. Participants at the March 2005 NDAC meeting acknowledged the difficulty in designing clinical trials to demonstrate the impact of health care antiseptics on rates of infection where numerous factors contribute to hospital-acquired infections, and therefore, would need to be controlled for in the design of these types of studies. Participants at the March 2005 NDAC meeting recommended that manufacturers perform an array of trials to look simultaneously at the effect on the surrogate endpoint and the clinical endpoint to try to establish a link between the surrogate and clinical endpoints, but provided no guidance on possible study designs. At the time, participants at the March 2005 NDAC meeting agreed that there were currently no clinical trials presented that showed a definitive clinical benefit for a health care antiseptic. However, recently, using an active comparator, Tuuli et al. demonstrated fewer infections following caesarean section with use of an approved patient preoperative health care antiseptic (Ref. 6). Otherwise, we have seen very few examples of well-controlled studies of this type to date.
Participants at the March 2005 NDAC meeting also believed it would be unethical to perform a hospital trial using a vehicle control instead of an antiseptic given the concerns with performing placebo-controlled studies on patients (Ref. 3). The inclusion of such control arms in a clinical outcome study conducted in a hospital setting could pose an unacceptable health risk to study subjects (hospitalized patients and health care providers). In such studies, a vehicle or negative control would be a product with no antimicrobial activity. The use of vehicle or saline (a negative control) in a hospital setting (a setting with an already elevated risk of infections) could increase the risk of infection for both health care providers and their patients. For these reasons, we continue to find that the use of clinical simulation studies relying on surrogate endpoints to evaluate the effectiveness of health care antiseptics is the best means available of assessing the effectiveness of health care antiseptic products.
(Comment 11) Given the ethical concerns with performing clinical trials in a health care setting, one comment urged FDA to evaluate natural experiments that have already occurred (e.g., hospital systems that switched away from chemical antiseptics in hand washes) when making a final monograph decision. The comment also stated that, while the clinical simulation studies provide useful information about one possible route through which bacterial illnesses are passed in a health care setting, as currently designed these studies do not study the complex microflora of the hospital environment, which is home to a wide range of bacterial populations. The comment said that the bactericidal effectiveness of the active ingredients is only partially achieved with the in vitro testing. The comment explained that, in addition to the MIC and time-kill testing, the in vitro tests for health care antiseptics could mirror the “worst-case” real-world assumptions. Clinical isolates that closely represent worst-case hospital or health care microbial populations (e.g., large numbers of multi-drug resistant bacterial strains) could be highly useful in determining Start Printed Page 60486the effectiveness of an active ingredient under real-world conditions. The comment stated that worst-case assumptions could include patient-derived isolates from cases involving isolation due to multi-drug resistance or isolates from frequently contaminated surfaces within a hospital or health care setting (e.g., door knobs, soap dispensers); and that this type of testing could be expanded into “clinical simulation” studies by measuring log reduction of bacterial counts on hands contaminated under actual health care conditions.
(Response 11) We believe that applying health care-associated high risk microbial pathogens (e.g., methicillin-resistant Staphylococcus aureus) during clinical simulation studies raises the ethical and study design issues we have discussed in this rulemaking. Currently, no historical data have been submitted to the docket that address or evaluate the effectiveness of health care antiseptic active ingredients in health care settings. Also, we are not aware of any health care personnel hand wash antiseptic that has been replaced with the use of plain soap and water in the hospital setting, and no such data have been submitted to the docket. Moreover, as explained in this rulemaking, participants at the March 2005 NDAC meeting believed that it would be unethical to perform hospital trial studies using a vehicle control, such as plain soap and water, instead of an antiseptic.
In addition, the standard infection control guidance broadly implemented by CDC (Refs. 7 and 8), which involves measures such as gloving, hand hygiene, patient-to-patient contact, and waste disposal, makes it difficult to design an adequate clinical study (Ref. 9).
Moreover, the in vitro testing required for proof of effectiveness against microorganisms (80 FR 25166 at 25177 to 25178), is already intended to characterize the activity (broad spectrum) of the antimicrobial ingredient. The American Type Culture Collection (ATCC) strains we reference in the 2015 Health Care Antiseptic PR for the in vitro testing are chosen to represent a broad spectrum of bacteria that present a challenge to antisepsis and are the principal bacterial pathogens encountered in hospital settings. The clinical simulation studies described in the 2015 Health Care Antiseptic PR are based on the premise that bacterial reductions achieved using tests that simulate conditions of actual use for each OTC health care antiseptic product category reflect the bacterial reductions that would be achieved under such conditions of use.
2. Log Reduction Testing Criteria
(Comment 12) Multiple comments were submitted to the 2015 Health Care Antiseptic docket on the in vivo testing criteria that use bacterial log reductions for determining the effectiveness of active ingredients used in health care antiseptic products. One comment stated that single application testing and increased log reduction for health care personnel hand rubs is not supported by scientific evidence and that current gaps exist within the peer-reviewed literature. The comment recommended that the Agency not change the testing requirements for the health care personnel hand rub products because alcohol-based hand rubs are used millions of times a day across the United States in all health care facilities. The comment also asserted that the recommended changes to the testing requirements by FDA could result in the unavailability of hand hygiene products to the clinicians who utilize them daily to prevent the transmission of health care associated infections to patients. One comment also asserted that FDA should retain the effectiveness criteria proposed for surgical hand scrubs identified in the 1994 TFM for single applications only.
Several comments also asserted that FDA should retain the effectiveness criteria proposed in the 1994 TFM for health care personnel hand wash and rub products as 2 log10 after a single application. The comments argued that the proposed 2.5 log10 reduction with a 70 percent success criterion for health care personnel hand wash products would be unattainable even by current FDA-approved products. In addition, several comments suggested that FDA adopt effectiveness criteria for in vivo effectiveness testing of active ingredients in surgical hand rubs and scrubs of a 1 log10 reduction within one minute after the first application procedure with no return to baseline within 6 hours.
Several comments also asserted that it is inappropriate to propose a 30-second contact time for patient preoperative skin preparations. The comments argued that most active ingredients for use in patient preoperative skin preparations would be unable to make the log reduction effectiveness criteria at 30 seconds. The comments asserted that, although it may be possible for some patient preoperative skin preparation products to make the log reduction effectiveness criterion and that it may be possible for some patient preoperative skin preparation products to make the 70 percent success rate for abdomen, no products can make the 70 percent success rate for the groin area at 30 seconds. One comment agreed with the 30-second time point, but argued that sampling should include a time point after the drying time is completed according to the directions. The comment stated that, in the proposed amendment to the 1994 TFM, it is unclear whether the antiseptic would be tested 30 seconds after application and while still wet, potentially resulting in efficacy compromise. The comment asserted that FDA should allow the product to fully dry before collecting 30-second time point efficacy testing, especially with topical skin antiseptics, because it is important that the skin be fully dry to achieve maximum efficacy and also to minimize potential skin irritation associated with use. Similarly, another comment asserted that, when referring to time points after product application for patient preoperative skin preparation, it should be explicitly stated that “after product application” means “product application plus required dry time.” Several comments also stated that the proposed 10-minute application period identified in the 1994 TFM is more representative of current clinical application practices.
(Response 12) As described in the 2015 Health Care Antiseptic PR, we proposed revisions to the log reduction criteria for health care personnel hand washes and rubs, and for surgical hand scrubs and rubs based on the recommendations of the March 2005 NDAC meeting and comments to the 1994 TFM that argued that the demonstration of a cumulative antiseptic effect for these products is unnecessary (80 FR 25166 at 25178). We agreed that the critical element of effectiveness is that a product must be effective after the first application because that represents the way in which health care personnel hand washes and rubs and surgical hand scrubs and rubs are used. Given that we were no longer requiring a cumulative antiseptic effect, the log reduction criteria were revised to reflect this single product application and fall between the log reductions previously proposed for the first and last application. Accordingly, we continue to find that the log reduction criteria for these products should be applied to a single application of the product rather than to multiple applications of the product.
Moreover, in the 2015 Health Care Antiseptic PR, we also proposed that patient antiseptic skin preparations (i.e., patient preoperative and preinjection skin preparations) be able to Start Printed Page 60487demonstrate effectiveness at 30 seconds because we believed that injections and some incisions are made as soon as 30 seconds after skin preparation (80 FR 25166 at 25178). In vivo studies are based on the premise that bacterial reductions achieved using tests that simulate conditions of actual use for each health care antiseptic category reflect the bacterial reductions that would be achieved under conditions of such use. Accordingly, we find that the effectiveness criteria for patient antiseptic skin preparations (i.e., patient preoperative and preinjection skin preparations) should continue to include the 30-second sampling time point. Also, we find that the 10-minute sampling time point proposed in the 1994 TFM should also be included in the effectiveness criteria as a time point option for patient preoperative skin preparations. These products should be tested at the 30-second or 10-minute sampling time point after drying, according to the labeled directions for use. For patient preinjection skin preparations, however, the 10-minute sampling time point should not be a time point option. Patient preinjection skin preparations should be tested at the 30-second time point only.
Based on comments submitted on the 2015 Health Care Antiseptic PR and the Agency's further evaluation of additional data, we have updated the underlying statistical analysis related to the log reduction criteria for classifying health care antiseptic active ingredients as GRAE (Refs. 10, 11, 12, 13, 14, and 15).
In the 1994 TFM, FDA recommended that the general effectiveness of antiseptics be assessed in a number of ways, including conducting clinical simulation studies with the surrogate endpoint of the number of bacteria removed from the skin. In the 2015 Health Care Antiseptic PR, FDA made revisions to the effectiveness criteria set forth in the 1994 TFM, while continuing to recommend that bacterial log reduction studies be used to demonstrate that an active ingredient is GRAE for use in a health care antiseptic product. FDA recommended that these bacterial log reduction studies: (1) Include both a negative control (test product vehicle or saline solution) and an active control; (2) have an adequate sample size to show that the test product is superior to its negative control; (3) incorporate the use of an appropriate neutralizer and a demonstration of neutralizer validation; and (4) include an analysis of the proportion of subjects who meet the recommended log reduction criteria based on a two-sided statistical test for superiority to negative control and a 95 percent confidence interval approach (80 FR 25166 at 25178 to 25179). FDA also recommended that the success rate or responder rate of the test product be significantly higher than 70 percent. This meant that the lower bound of the 95 percent confidence interval for the proportion of subjects who met the log reduction criteria was expected to be at least 70 percent.
Consistent with the 1994 TFM and 2015 Health Care Antiseptic PR, we find that bacterial log reduction studies should continue to be used to demonstrate that an active ingredient is effective for use in a health care antiseptic product. Also consistent with the 2015 Health Care Antiseptic PR, subjects should be randomized to a three-arm study: Test, active control, and negative control. However, based on comments submitted on the 2015 Health Care Antiseptic PR and the Agency's further evaluation of additional data, we are updating the statistical analysis related to the log reduction criteria for classifying health care antiseptic active ingredients as GRAE. Also, as we explain in section V.B.4, we include separate effectiveness criteria for patient preinjection skin preparations to more accurately reflect the actual use of these products. We also clarify, for patient preoperative skin preparations and patient preinjection skin preparations, that the sampling time point commences after the applied product dries.
The updated analysis is designed to assess whether the average treatment effects (ATE) across subjects meet indication-specific conditions of superiority and non-inferiority, rather than whether the percentage of subjects who meet an indication-specific threshold significantly exceeds 70 percent. More specifically, the updated analysis estimates the ATE from a linear regression of post-treatment bacterial count (log10 scale) on the additive effect of a treatment indicator and the baseline or pre-treatment measurement (log10 scale). In the conditions below, the ATE of the test product compared to the negative control is defined as the contrast of treatment effect of negative control minus the treatment effect of the test drug in the linear regression. Likewise, the ATE of the active control compared to the test product is defined as the contrast of treatment effect of test product minus the treatment effect of the active control in the linear regression.
Superiority to negative control by a specific margin is needed because our evaluation suggests that application of a negative control, whether test product's vehicle or saline, may exhibit some minimal antimicrobial properties. Thus, using superiority to negative control by those margins will help ensure that we can appropriately assess the effectiveness of the deferred antimicrobial products. The margins we identify in this section were derived from review and analysis of existing data, and may be revised as data gaps on deferred antimicrobial products are filled. Because of existing data gaps, we also require the deferred ingredient to show non-inferiority to active controls by a 0.5 margin (log10 scale).
Accordingly, based on the updated analysis, the bacterial log reduction studies used to assess whether an active ingredient is effective for use in health care antiseptics should include the following:
- The test product should be non-inferior to an FDA-approved active control with a 0.5 margin (log10 scale). That is, we expect the upper bound of the 95 percent confidence interval of the ATE of the active control compared to the test product to be less than 0.5 (log10 scale). An active control is not intended to validate the study conduct or to show superiority of the test drug product but to show that the test drug product is not inferior. Non-inferiority to active control should be met at the following area and times for the respective health care antiseptic indications:
○ Patient preoperative skin preparation:
Per square centimeter on abdominal site within 30 seconds after drying, or within 10 minutes after drying
Per square centimeter on groin site within 30 seconds after drying, or within 10 minutes after drying
○ Patient preinjection skin preparation: Per square centimeter on a dry site (i.e., forearm, abdomen, or back) within 30 seconds after drying
○ Health care personnel hand wash: On each hand within 5 minutes after a single wash
○ Health care personnel hand rub: On each hand within 5 minutes after a single rub.
○ Surgical hand scrub: On each hand within 5 minutes after a single scrub
○ Surgical hand rub: On each hand within 5 minutes after a single rub
- The test product should be superior to the vehicle control by an indication-specific margin. That is, we expect the lower bound of the 95 percent confidence interval of the ATE of the test product compared to the vehicle control to be greater than the indication-specific margin. In cases where the vehicle cannot be used as a negative Start Printed Page 60488control, nonantimicrobial soap or saline solution can be used. Based on our evaluation of the existing data, the following indication-specific superiority margin should be met by the deferred ingredients for the respective health care antiseptic indications:
○ Superiority margin of 1.2 log10 for patient preoperative skin preparation
per square centimeter on abdominal site within 30 seconds after drying, or within 10 minutes after drying
per square centimeter on groin site within 30 seconds after drying, or within 10 minutes after drying
○ Superiority margin of 1.2 log10 for patient preinjection skin preparation per square centimeter on a dry site (i.e., forearm, abdomen, or back) within 30 seconds after drying
○ Superiority margin of 1.2 log10 for health care personnel hand wash on each hand within 5 minutes after a single wash
○ Superiority margin of 1.5 log10 for health care personnel hand rub on each hand within 5 minutes after a single rub
○ Superiority margin of 0.5 log10 for surgical hand scrub on each hand within 5 minutes after a single scrub
○ Superiority margin of 1.5 log10 for surgical hand rub on each hand within 5 minutes after a single rub
As discussed in more detail in section V.D.4, we believe that persistence of antimicrobial effect is an important attribute for health care antiseptic products, and in particular for patient preoperative skin preparations, surgical hand scrubs, and surgical hand rubs. To show persistence of effect for these health care antiseptic indications, the 6 hours post-treatment measurement should be lower than or equal to the baseline measurement for 100 percent of the subjects in each indication and body area tested.
Moreover, for the deferred ingredients, a minimum sample size of 100 subjects per treatment arm should be included for each indication. This sample size will ensure that ATE will be estimated precisely for the deferred ingredients and can be used for future reference in final product monographs. Exact sample size can be based on the margins for non-inferiority and superiority as well as an assessment of variability. In addition, two adequate and well-controlled clinical simulation pivotal studies should be conducted for each indication at two separate independent laboratory facilities by independent principal investigators.
3. Baseline Bacterial Count
(Comment 13) Several comments asserted that the Agency does not specify a minimum baseline bacterial count for subject eligibility in the clinical simulation studies and that the 1994 TFM is vague with regard to baseline values. The 1994 TFM states only that sites are to possess bacterial populations large enough to allow demonstrations of bacterial reduction of up to 2 log10 per square centimeter on dry skin sites and 3 log10 per square centimeter on moist sites (59 FR 31402 at 31450). One comment urged FDA to use baseline values for patient preoperative skin preparations that follow the American Society for Testing and Materials (ASTM) [3] method E1173, which is more specific and states that the bacterial baseline population should be at least 3 log10 per square centimeter on moist skin sites and at least 2 log10 greater than the detection limit on dry skin sites. Several comments also stated that it was challenging to find subjects who have resident bacterial counts high enough to be eligible for these studies.
(Response 13) We do not specify a minimum baseline bacterial count for subject eligibility in the clinical simulation studies; however, the test sites should possess bacterial populations large enough to meet the updated statistical criteria as explained in section III.D.2. We do not specify a minimum baseline bacterial count because, as explained in section III.D.2, the ATE is used to demonstrate effectiveness. Rather than using only a change from baseline, each criterion (groin site and abdomen site) uses the ATE, an estimated difference of the effect of two treatments correcting for baseline count. Manufacturers are encouraged to select subjects with baseline counts significantly higher than the expected log reductions achieved during the testing (i.e., high enough to allow for a positive residual of bacterial burden after the use of the active control and the test product). This selection will ensure that there is a high enough bacterial count at baseline to assess the full effectiveness of both the active control and the product under evaluation. Likewise, a bacterial burden so low that it is depleted readily both by the vehicle (or negative control) and by the test product, will not allow for an assessment of the effectiveness of that test product because the outcome would equally be zero and it will not be possible to measure the difference in log reduction between the test product and negative control. The number of viable microorganisms recovered from the skin of each subject at baseline should be provided in the final study report. In addition, given the updated statistical analysis criteria outlined in section V.D.2, it is unnecessary to apply the baseline values for patient preoperative skin preparations that follow the ASTM E1173 method.
Moreover, if manufacturers find it challenging to recruit subjects who have resident bacterial counts high enough to be eligible for these studies, we recommend the use of the back as an alternate dry test site, rather than using the arm. We do not recommend the use of an occlusive dressing (sterile gauze). Covering the test sites has the potential to change the make-up of the microbial population. Therefore, the use of occlusion may not provide an accurate assessment of how effective the product will be under actual use conditions.
4. Persistence
(Comment 14) One comment stated that current infection control procedures make persistence of antimicrobial activity for surgical hand scrub and patient preoperative skin preparations irrelevant. The comment asserted that persistence of effect may, in fact, be a negative attribute for these products because it may cause irritation. The comment suggested that the Agency place more emphasis on the mildness of these products rather than the persistence of these products. Another comment agreed with the Agency's requirement that patient preoperative skin preparations and surgical scrubs have a persistent antimicrobial effect. Another comment contended that the Agency's statement about the need for persistence of effect for patient preoperative hand scrubs lacks substantiating data. Another comment stated that the concept of persistence of antimicrobial activity is not consistent for surgical scrub and patient preoperative skin preparations, nor is it consistent with clinical practice. The comment asserted that the testing requirements for a patient preoperative skin preparation limit the definition of persistence to 6 hours of sustained activity after each product use. The comment recommended that persistence for surgical hand scrub products be defined as sustained activity of the antimicrobial formulation for a period of 6 hours after product use. Another comment asserted that persistence should not be required for any of the health care indications.
(Response 14) In the 1994 TFM, we described the importance of persistence as a characteristic of antiseptic drug Start Printed Page 60489products. We agreed with the Advisory Review Panel on OTC Miscellaneous External Drug Products' finding that persistence, defined as prolonged activity, is a valuable attribute that assures antimicrobial activity during the interval between washings and is important for a safe and effective health care personnel hand wash. We agreed that a property such as persistence, which acts to prevent the growth or establishment of transient microorganisms as part of the normal baseline or resident flora, would be an added benefit (59 FR 31402 at 31407). Accordingly, we proposed to include the persistence requirement in the definitions of patient preoperative skin preparations and surgical hand scrubs because we believe that persistence of antimicrobial effect would suppress the growth of residual skin flora not removed by preoperative prepping as well as transient microorganisms inadvertently added to the operative field during the course of surgery and reduce the risk of surgical wound infection. Specifically, we proposed to define patient preoperative skin preparation to be a fast acting, broad spectrum, and persistent antiseptic containing preparations that significantly reduce the number of micro-organisms on intact skin, and we proposed to define surgical hand scrub drug products to be an antiseptic containing preparation that significantly reduces the number of microorganisms on intact skin; it is broad spectrum, fast acting, and persistent (59 FR 31402 at 31442). In addition, although we do not require persistence for health care personnel hand washes, we did propose to retain the words “if possible, persistent” in the definition of health care personnel hand wash (59 FR 31402 at 31442).
FDA continues to believe that persistence of antimicrobial effect is an important attribute because it can suppress the growth of residual skin flora, as well as transient microorganisms not removed by preoperative prepping or hand scrubbing. FDA is also aware that the donning of surgical gloves may produce a rapid increase in microbial count on the hands (Refs. 16, 17, and 18), even after use of a surgical hand antiseptic product, which is another reason why persistence of effect is a critical characteristic for antiseptic products. Accordingly, we find that persistence is a requirement for surgical hand scrubs, surgical hand rubs, and patient preoperative skin preparations. We find that these antimicrobial products must be fast-acting and consist of broad spectrum, persistent antiseptic-containing preparations that significantly reduce the number of microorganisms on intact skin. As discussed in section V.D.2 of this final rule, to show the persistence of effect for these health care antiseptic indications, the 6 hours post-treatment measurement should be lower than or equal to the baseline measurement for 100 percent of subjects for each indication and body area tested.
5. Controls
(Comment 15) Several comments objected to the use of controls because we do not specify what positive control material to use in the effectiveness studies. One comment contended that, because the Agency does not specify the control product, the test results will differ depending on the effectiveness of the positive control. Another comment recommended that we convene an expert panel to develop standard positive controls. They cite the trend, on a worldwide basis, to identify and adopt standardized testing procedures. They believe it would be far better for the international harmonization effort if a standard chemical, rather than a specific product or commercial formulation, was used as the control. For these reasons, the comment recommended that the positive control should be a standard chemical that can be produced on a global basis and will perform consistently and reproducibly.
Other comments requested that we clarify how to interpret the results of the positive control. One comment asked if our standard is meeting the required log reduction, superiority to the positive control, or both. Another comment pointed out that the Agency does not define the criterion for an acceptable outcome for the positive control. For instance, the comment states that it is unclear if an 80 percent success rate in the positive control for a surgical hand scrub would be acceptable and if so, whether the new treatment could be 20 percent less successful than the positive control and still be equivalent. For health care personnel hand washes, they assert that it is not clear if the control must meet the requirements of 2 and 3 log10 reduction at the lower 95 percent confidence interval limit or an average. The comment requested that FDA specify criteria for validity of the study in terms of the positive control and criteria for concluding that a test material is effective in terms of equivalence to the positive control. One comment noted that the Agency's proposed patient preoperative skin preparation treatment application procedure does not include any reference to the active control sites.
Several comments agreed that the Agency's proposed changes to the in vivo efficacy testing will reflect more accurately the real world use of topical antiseptic drug products. The comments requested that the Agency provide a validated “gold standard” for use as an active control. One comment stated that it is appropriate that GRAS/GRAE active ingredients would serve as the active control for any effectiveness studies required for final formulations. For example, the comment explained that alcohol at the concentration and application instructions evaluated in the pivotal studies to help establish GRAS/GRAE status would become the active control for effectiveness studies involving alcohol-based final formulations. This would be more appropriate than using an FDA-approved product for the active control, particularly for alcohol-based hand sanitizer products where the only FDA-approved drug is a dual-active product.
(Response 15) We do not define a specific positive control material to use in the effectiveness studies in this final rule, but we do recommend the use of an appropriate FDA-approved NDA antiseptic as the positive control (i.e., active control) when conducting the effectiveness testing of health care antiseptic active ingredients. We recognize that many countries have adopted standard chemicals for their active controls. However, we still believe that we cannot define a specific active control product for the following reasons:
- We do not have sufficient data to choose a specific universal active control product that will be appropriate for all test formulations or active ingredients.
- Changes to the formulation or manufacturing of the chosen active control product might affect its activity in future studies. Consequently, products tested against the modified active control might not be held to the same standards as products tested previously.
Although we do not identify a specific control product, we do identify test criteria for the active control. As described in section V.D.2, we recommend the use of non-inferiority of the test product to an FDA-approved active control by a margin of 0.5 (log10 scale). That is, we expect the upper bound of the 95 percent confidence interval of the ATE of the active control compared to the test product to be less than 0.5 (log10 scale). An active control is not intended to validate the study conduct or show superiority of the test Start Printed Page 60490drug product, but to show that the test drug product is not inferior.
In addition, we recommend the use of an active control product of the same type as the test product. For example, if the test product is a leave-on surgical hand antiseptic, then an FDA-approved leave-on surgical hand antiseptic should be used as the active control rather than a rinse-off surgical hand antiseptic. We believe it is more appropriate to compare similar types of products.
(Comment 16) One comment stated that a vehicle typically refers to the product formulated without the active ingredient. The comment recommended that the term “vehicle” be replaced with the term “negative control.” Another comment requested that FDA clarify whether testing of the vehicle is required.
(Response 16) We recognize that the term “negative control” may be broader than the term “vehicle,” and we agree that the term “vehicle” should be replaced with the term “negative control” where applicable. As discussed in section V.D.2, we recommend that the effectiveness testing study design for health care antiseptic active ingredients include a negative control arm, which is used as a comparator for the test product. The appropriate negative control to be used in the studies is the test product's vehicle, which we interpret to be the same product being tested, without the active ingredient included, and therefore, best represents the independent contribution of the antiseptic active ingredient. Because the same directions for use will apply to the negative control and the test product, this should account for any potential mechanical removal of microorganisms, which occurs during the rubbing, scrubbing, wiping, or rinsing process, independent of the active ingredient effect. If there is a scientific reason why testing a product using its vehicle as a negative control is not feasible, discussions can be had with FDA to determine whether the use of an alternative negative control, such as a saline solution or nonantimicrobial soap (for health care personnel and surgical hand antiseptics), may be acceptable.
We note that the testing described in this document pertains to single active ingredients. Manufacturers should contact us if, in the future, they would like to develop a fixed-combination health care antiseptic drug product.
6. In Vitro Testing
(Comment 17) One comment outlined the Agency's proposed requirements listed in the 2015 Health Care Antiseptic PR (80 FR 25166 at 25177 to 25178) for an evaluation of the spectrum and kinetics of antimicrobial activity of a health care antiseptic as including the following:
- A determination of the in vitro spectrum of antimicrobial activity against recently isolated normal flora and cutaneous pathogens;
- Minimum inhibitory concentration (MIC) or minimum bactericidal concentration (MBC) testing of 25 representative clinical isolates and 25 reference strains of each of the microorganisms listed in the 1994 TFM; and
- Time-kill testing of each of the microorganisms listed in the 1994 TFM to assess how rapidly the antiseptic active ingredient produces its effect. The dilutions and time points tested should be relevant to the actual use pattern of the final product.
The comment requested that we confirm that the first bullet is meant to describe what will be learned from the studies outlined in the last two bullets because they do not recognize the first bullet as an actual study. The comment also asked for confirmation that the emergence of resistance testing is no longer a requirement.
Another comment stated that the Agency has proposed in vitro testing of 1,150 microorganisms (25 clinical isolates and 25 reference isolates for 23 microorganisms). The comment argued that the Agency's suggestion that previous tests of the same or similar strains are no longer valid is arbitrary and that the requirement for new repeated tests is unduly burdensome. The comment asserted that the proposed number of clinical and reference isolates far exceeds the number required for FDA-approved hand hygiene products, which have successfully completed the review process. The comment recommended that organisms of current clinical value as well as recent clinical isolates be utilized to better assess the in vitro efficacy of these active ingredients. Another comment similarly asserted that the microorganisms identified by FDA for antimicrobial activity testing do not include pathogens that are relevant to current health care settings; the comment argued that the list should include Methicillin-resistant Staphylococcus aureus, Methicillin-resistant Staphylococcus epidermidis, Vancomycin-resistant Enterococcus; Enterococcus faecalis and Enterococcus faecium). Another comment proposed that FDA should consider adequate justifications for testing fewer than the identified strains for organisms where 25 clinical isolates and/or 25 standard strains are not available for screening active ingredients.
(Response 17) We agree that the determination of the in vitro spectrum of antimicrobial activity against recently isolated normal flora and cutaneous pathogens is meant to describe what will be learned from the MIC and/or MBC and time-kill studies and is not intended to be a separate study. With regards to testing for the emergence of resistance, we are requiring resistance testing for three of the six deferred active ingredients—benzalkonium chloride, benzethonium chloride, and chloroxylenol (Refs. 10, 11, 12, 13, 14, and 15). However, we are not requiring resistance testing for the other three deferred active ingredients—ethyl alcohol, isopropyl alcohol, and povidone-iodine (see section V.D.2).
In addition, we disagree that we are suggesting that previous tests of the same or similar strains are no longer valid. In the 2015 Health Care Antiseptic PR, we proposed the option of assessing the MBC as an alternative to testing the MIC. We also reiterated our proposal that the evaluation of the spectrum and kinetics of antimicrobial activity of health care antiseptic active ingredients should include MIC (or MBC) testing of 25 representative clinical isolates and 25 reference (e.g., ATCC) strains of each of the microorganisms listed in the 1994 TFM, in addition to the other proposed requirements. In the 2015 Health Care Antiseptic PR, we noted that, despite the fact that the in vitro data submitted to support the effectiveness of antiseptic active ingredients were far less extensive than proposed in the 1994 TFM, manufacturers may have data from their own product development programs which they have not submitted to the docket and/or that published data may have become available that would satisfy some or all of the data requirements (80 FR 25166 at 25178).
As we explained in the 2015 Health Care Antiseptic PR, we agree that the in vitro testing proposed in the 1994 TFM is not necessary for testing every final formulation of an antiseptic product that contains a GRAE ingredient (80 FR 25166 at 25177). However, we continue to believe that a GRAE determination for health care antiseptic active ingredients should be supported by adequate in vitro characterization of the antimicrobial activity of the ingredient. We note that, for the six deferred active ingredients, the Agency is reviewing proposed protocols for the safety and effectiveness studies, including the list of organisms for the time-kill testing and MIC/MBC testing, which may include additional resistant organisms that are relevant to current health care settings.Start Printed Page 60491
7. American Society for Testing and Materials Standards
(Comment 18) Several comments proposed that the Agency recognize specific ASTM protocols as standardized test methods for demonstrating that an active ingredient is GRAE for use in health care antiseptics and demonstrating effectiveness for final product formulations. These ASTM test methods include the ASTM E1174 “Standard Test Method for the Evaluation of the Effectiveness of Health Care Personnel Handwash Formulations”; the ASTM E2755-10 “Standard Test Method for Determining the Bacteria-Eliminating Effectiveness of Hand Sanitizer Formulations Using Hands of Adults”; the ASTM E1115-11 “Standard Test Method for Evaluation of Surgical Hand Scrub Formulations”; the ASTM E1173-15 “Standard Test Method for Evaluation of Preoperative, Precatheterization, or Preinjection Skin Preparations”; the ASTM E1054 “Standard Test Methods for Evaluation of Inactivators of Antimicrobial Agents”; the ASTM E2783 “Standard Test Method for Assessment of Antimicrobial Activity for Water Miscible Compounds Using a Time-Kill Procedure”; and the Clinical and Laboratory Standards Institute M07-A10 “Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically.”
(Response 18) For purposes of the six deferred active ingredients, we have reviewed these test methods and believe they may be useful to help establish GRAE status for the health care antiseptic products for their respective indications. We are currently discussing with manufacturers and trade organizations that requested the deferrals how these test methods may be used to meet the current effectiveness criteria.
Testing requirements for final formulation, however, are not addressed in this final rule because none of the active ingredients subject to this final rule have been found to be GRAE for use in health care antiseptic products. The testing requirements for final formulation of these products containing the six deferred active ingredients will be addressed after a decision is made regarding the monograph status of those ingredients.
E. Comments on Safety and FDA Response
1. Need for Additional Safety Data
(Comment 19) One comment supported FDA's proposal to require additional safety data for the health care antiseptic active ingredients. The comment agreed that more testing is needed to support a GRAS determination for these active ingredients. Other comments, however, asserted that the safety testing proposed in the 2015 Health Care Antiseptic PR for active ingredients used in health care antiseptics is unnecessary and burdensome. The comments asserted that FDA has not provided data to justify that additional safety data are needed for these ingredients to make a GRAS determination and stated that the extensive historical use of these products should serve as proof of the products' safety and effectiveness.
Another comment stated that FDA must document how the systemic absorption levels of active ingredients from the use of health care antiseptics differ from FDA's previous assessment of the safety of these ingredients. The comment asserted that, given the lack of information on FDA's current position on the specific details regarding risk assessment, FDA should consider in vitro data and dose-extrapolation data.
Another comment suggested that long-term systemic exposure to active ingredients used in health care antiseptics could be reduced if the efficacy standards for these products were decreased because lower dose products could be formulated.
(Response 19) We continue to believe that the additional safety data outlined in the 2015 Health Care Antiseptic PR are necessary to support a GRAS classification for the health care antiseptic active ingredients. As was explained in the 2015 Health Care Antiseptic PR, several important scientific developments that affect the safety evaluation of the health care antiseptic active ingredients have occurred since FDA's 1994 evaluation. New data and information on the health care antiseptic active ingredients raise concerns regarding potential risks from systemic absorption and long-term exposure, as well as development of bacterial resistance related to widespread antiseptic use (80 FR 25166 at 25167). Data that meet current safety standards are needed for FDA to conduct an adequate safety evaluation to ensure that health care antiseptic active ingredients are GRAS. Moreover, as previously explained in this document, the September 2014 NDAC meeting participants discussed FDA's proposed revisions to the safety data requirements and agreed that these requirements were appropriate to demonstrate that a health care antiseptic active ingredient is GRAS. Participants at the September 2014 NDAC meeting further concluded that these safety standards are reasonable and considered them to be minimal safety standards for currently available, as well as future healthcare antiseptic products (Ref. 19).
Moreover, the long history of use of a drug product is not sufficient to demonstrate the safety of the product. In the case of antiseptic products, the Agency has requested safety data in both the 1994 TFM and the 2015 Health Care Antiseptic PR in order to finalize the antiseptic rules. Relying solely on adverse event reporting cannot fill data gaps regarding risks such as reproductive toxicity or carcinogenicity. As an example, phenolphthalein was an OTC product with a long history of use as a laxative, but when animal studies were conducted, evidence of carcinogenicity was detected. The April 30, 1997, FDA Center for Drug Evaluation and Research (CDER) Carcinogenicity Assessment Committee (CAC) meeting concluded that there was supportive evidence indicating that phenolphthalein may be carcinogenic through a genotoxic mechanism. FDA concluded “phenolphthalein caused chromosome aberrations, cell transformation, and mutagenicity in mammalian cells. Because benign and malignant tumor formation occurs at multiple tissue sites in multiple species of experimental animals, phenolphthalein is reasonably anticipated to have human carcinogenic potential.” This conclusion led to the removal of phenolphthalein from the market (64 FR 4535, 4538) (Ref. 20).
Finally, in this context, the safety data required to make a final GRAS determination on active ingredients used in health care antiseptic products would remain the same even if FDA determined that the data requirements necessary to make a GRAE determination should be changed.
(Comment 20) Several comments also stated that the additional testing requirements could cause disruptions of the availability of health care antiseptics for clinical use. One comment urged the Agency to fully consider the consequences of the additional testing requirements, especially at a time when hand hygiene is considered to be the cornerstone for preventing the spread of pathogenic organisms in health care settings.
(Response 20) We agree that health care antiseptic products are an important component of infection control strategies in health care settings and remain the standard of care to prevent illness and the spread of infections (Refs. 7 and 8). As we emphasized in the 2015 Health Care Antiseptic PR, our proposal for more safety and effectiveness data for health Start Printed Page 60492care antiseptic active ingredients does not mean that we believe that health care antiseptic products containing these ingredients are ineffective or unsafe. However, data that meet current safety requirements are still needed to support a GRAS determination for these active ingredients used in health care antiseptic products.
We do not believe that these additional testing requirements will disrupt the availability of health care antiseptics for clinical use. As explained in the 2015 Health Care Antiseptic PR, we provided a process for seeking an extension of time to submit the required safety and/or effectiveness data if needed (80 FR 25166 at 25169). As discussed in this document, we have deferred further rulemaking on six active ingredients used in OTC health care antiseptic products to allow for the development and submission of new safety and efficacy data. Although in this final rule we find that the 24 non-deferred active ingredients are not GRAS/GRAE for use in OTC health care antiseptic products, health care antiseptic drug products that have been approved under an NDA or that contain one or more of the six deferred active ingredients still continue to be available.
Accordingly, we do not believe that the additional testing requirements will cause a disruption in the availability of OTC health care antiseptic products.
(Comment 21) Another comment asserted that FDA's reasons for requesting additional safety data are flawed. The comment stated that FDA should analyze all existing hazard data and consider the extent of human or environmental exposure as part of the process for deciding the nature and extent of hazard data required to understand potential safety concerns. The comment asserted that data generation based on an understanding of human exposure prevents the irresponsible use of laboratory animals and waste of resources necessary to generate toxicology data that will not further inform potential safety decisions.
The comment also contended that the safety data gaps cited by FDA for the ingredients in the 2015 Health Care Antiseptic PR (human pharmacokinetics, animal pharmacokinetics, carcinogenicity, reproductive toxicity, potential hormonal effects, and potential antimicrobial resistance) do not all have to be filled in order for FDA to make a GRAS determination. In support of its position, the comment cited FDA's presentation to the September 2014 NDAC meeting, and listed FDA's stated criteria associated with the GRAS standard, including: (1) A low incidence of adverse events when used as directed and in the context of warnings; (2) low potential for harm if abused under conditions of widespread availability; (3) significant human marketing experience; (4) and, adequate tests to show proof of safety, among other criteria. The comment stated that FDA is not taking into account the low incidence of adverse events associated with the use of antiseptic active ingredients and the overall acceptance of these products globally. The comment also mentioned that numerous scientific and regulatory bodies have performed exposure-driven risk assessments and have not required the types of human or animal data mentioned in the 2015 Health Care Antiseptic PR.
(Response 21) FDA presented the safety paradigm for OTC health care antiseptics at the September 2014 NDAC meeting (Ref. 21) where the Agency sought NDAC's advice about the type and scope of safety data needed for OTC health care antiseptic products. In FDA's presentation to NDAC, we explained that when evaluating a proposed monograph active ingredient, FDA applies the following regulatory standards, which are cited in 21 CFR 330.10(a)(4)(i):
- Safety means a low incidence of adverse reactions or significant side effects under adequate directions for use and warnings against unsafe use, as well as low potential for harm which may result from abuse under conditions of widespread availability.
- Proof of safety shall consist of adequate tests by methods reasonably applicable to show the drug is safe under the prescribed, recommended, or suggested conditions of use. This proof shall include, but not be limited to, results of significant human experience during marketing.
- General recognition of safety shall ordinarily be based upon published studies, which may be corroborated by unpublished studies and other data.
As FDA explained in its presentation, the proposed safety studies are necessary to provide data that are needed to support a GRAS determination for the health care antiseptic active ingredients. The NDAC unanimously agreed that the safety standards proposed by FDA are appropriate to support a GRAS determination for a health care antiseptic active ingredient. The NDAC also noted that the safety standards presented by FDA are reasonable minimal safety standards for the currently available antiseptics, as well as for products to be formulated in the future (Ref. 19) and are required to support a GRAS determination for these ingredients.
In terms of animal testing, the September 2014 NDAC meeting addressed the issue of the appropriateness of conducting animal studies to obtain safety data for health care antiseptic products (Ref. 4). We understand that animal use in tests for the efficacy and safety of human and animal products has been and continues to be a concern, and FDA continues to support efforts to reduce animal testing, particularly where new alternative methods for safety evaluation have been validated and accepted by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) regulatory authorities. To address this issue, we encourage manufacturers to consult with the Agency on the use of non-animal testing methods that may be suitable, adequate, validated, and feasible to fill important data gaps that cannot be filled with marketing experience alone. However, there are still many areas where non-animal testing has not been sufficiently developed as an alternative option and animal studies are still considered necessary to fill important safety gaps (Refs. 4 and 19).
2. MUsT Requirements
(Comment 22) One comment asserted that FDA should reconsider the need to conduct MUsTs to assess systemic exposures associated with extreme use applications. The comment stated that the clinical utility of this testing has not been firmly established and the methodology necessary to conduct this type of testing has yet to be clearly validated to establish its utility. The comment argued that these types of studies need significant further development and validation before considering them a reliable method for systemic absorption studies and further guidance from FDA is needed. The comment said that FDA should also consider the use of existing modeling methods as a means to assess potential systemic exposure to avoid unnecessary clinical testing of active ingredients where modeling is available in conjunction with animal data.
(Response 22) The MUsT paradigm has been used in the evaluation of topical dermatological agents approved in the United States since the early 1990s. It represents over 20 years of interactions with multi-national drug companies, during which time the study design has been refined into its current state. Moreover, the MUsT is a published methodology that has been Start Printed Page 60493presented at both national and international meetings. In addition, with respect to the six deferred active ingredients, FDA has been reviewing the MUsT protocol designs submitted by the manufacturers and trade organizations that have requested deferrals.
FDA also understands and recognizes the potential of pharmacokinetic (PK) and physiologically-based pharmacokinetic (PBPK) modeling. FDA has considered these options and concluded that the currently proposed alternatives, including in silico, in vitro, and PBPK modeling, are not adequately validated to be a substitute for the MUsT described in the 2015 Health Care Antiseptic PR. We also note that, going forward, in order to validate the PBPK or any other alternative modeling-based approach, one would need, as part of their validation, a direct performance comparison to a series of in vivo MUsTs as part of the process to demonstrate the comparability and reproducibility of the results between the tests. For these reasons, we find that results from a human PK MUsT are needed to support a GRAS determination for active ingredients used in health care antiseptic products.
(Comment 23) Another comment disagreed with FDA's position that the lack of pharmacokinetic data prevents FDA from calculating a margin of exposure for the risk assessment. The comment asserted that, although the safety evaluation of drugs may rely on correlating findings from animal toxicity studies to humans based on kinetic information in both species, safety evaluations for antiseptic ingredients in health care products are not based on kinetic information under standard international practice. Instead, the comment argued, safety evaluations are based on conservative assumptions of exposure and potential differences between species, and kinetic information is only required when use of these conservative assumptions fails to provide a sufficient margin of exposure. The comment stated that using these conservative and internationally accepted approaches, other scientific bodies and regulatory authorities have been able to complete the risk assessment for these types of ingredients in formulations with much greater levels of human exposure than these health care antiseptic uses. The European Commission Scientific Committee on Consumer Safety Guidance for the Testing of Cosmetic Substances and Their Safety Evaluation (8th Revision) was cited as a justification for this concept. Based on this reasoning, the comment asserted that FDA should not require additional animal testing unless the following conditions are met:
- Use of conservative approaches to calculate the margin of exposure is inadequate.
- The margin of exposure justifies the need for more data, but it is not possible to generate the data by non-animal approaches, such as using physiologically-based pharmacokinetic modeling, or through animal alternative test methods.
- There is perceived need for all active ingredients to have the same type of information.
(Response 23) Calculating the margin of exposure was one of the topics discussed at the September 2014 NDAC meeting (Refs. 4 and 19). At that time, the consensus reached was that these types of calculations are more informed when taking the results of the MUsT-acquired data and using that information along with the pharmacology/toxicology results in the calculation of the safety margin. We also note that the references the comments provided for the risk assessment strategies that are followed by other international agencies are for cosmetic ingredients rather than for drug products. Accordingly, the referenced guidance may be designed to address different concerns than those at issue here.
(Comment 24) Another comment stated that FDA should reconsider the concept of the MUsT and its value in determining the safety of health care antiseptic products. The comment said that the 2015 Health Care Antiseptic PR would require a MUsT to characterize maximum systemic exposure following health care antiseptic product use during the course of a work day or shift in health care settings. The comment stated that measured levels determined by the MUsT would establish the maximum systemic dose for the active ingredient in the particular antimicrobial product type, and the representativeness of the measured systemic active concentration would be dependent upon a number of variables associated with this trial, including the number of applications made per day or shift, the appropriate usage of the product, the concentration of active ingredient in the tested product, the sensitivity of the analytical method applied, and the extent to which the experimental protocol matches or approximates the actual usage of the product in the health care setting. The comment asserted that the use of the same product in different health care settings (e.g., out-patient clinics or offices vs. emergency rooms or operating rooms) can be expected to have different patterns of use.
The comment also argued that limitations exist in the practical conduct of a MUsT that influence and dictate what may be achieved by a specific protocol. The comment stated that practical requirements, for instance, the time needed to collect biological samples, or even to perform washing or application of the product, will dictate how many washes or applications are possible in a given time period regardless of what may be deemed desirable or required to evaluate perceived or empirical usage. As a result, the comment argued, the MUsT conditions described in the 2015 Health Care Antiseptic PR will result in assays that are very large and complex, and there is very little precedent to consult in the published literature. The comment also argued that the practical aspects of conducting a MUsT dictate what can reasonably be performed in terms of number of product applications, number of subjects, study arms, and timing. The comment asserted that if the defined, or desired, maximal use is not achievable in a MUsT and the resulting data do not meet the needs of the safety and risk assessment process, it is reasonable to question the utility, and expense, of conducting the study at all.
(Response 24) The MUsT intends to reflect the upper end of use expected in the real-world. Because the MUsT is designed to represent, as closely as possible, the maximal use of the health care antiseptic product under actual use conditions in the health care setting, the conduct of the trial itself should be feasible. The goal of the MUsT is to evaluate absorption under conditions of maximum use, so lower rates of application, different sites, and different frequency of application will be covered. As we also mentioned, with respect to the six deferred active ingredients, FDA is reviewing protocol designs for the respective deferred active ingredients.
(Comment 25) Another comment stated that, while data on the level of active ingredient in systemic circulation is arguably important for risk and safety assessment, it is not clear what any observed levels from MUsT may mean in this context in regards to risk and safety assessment. The comment argued that FDA has provided little guidance on how the MUsT data are used and that FDA has provided no data to indicate that there are any safety issues associated with any of the six active ingredients identified in the comment (alcohol, isopropyl alcohol, benzalkonium chloride, benzethonium Start Printed Page 60494chloride, povidone-iodine, and chloroxylenol). The comment also asserted that, while the MUsTs will provide information on active ingredient levels in systemic circulation, it fundamentally remains a pharmacokinetic study. As such, the comment argued, it is not apparent that results from a MUsT will provide data that could not be better determined by an alternative or otherwise validated and accepted approach.
(Response 25) We disagree with the comment's assertion that the Agency has not provided any data to indicate that there are safety issues associated with the six active ingredients identified in the comment, which are the six active ingredients we have deferred from this rulemaking. Based on known available data, including data submitted by the interested parties, FDA identified and summarized safety concerns and safety data gaps for the health care active ingredients at the September 2014 NDAC meeting (Refs. 4 and 21) and in the 2015 Health Care antiseptic PR (80 FR 25166 at 25179 to 25195).
Moreover, the MUsT approach was specifically discussed at the September 2014 NDAC meeting (Refs. 4, 19, and 21). Information on systemic exposure derived from the MUsTs is necessary to determine a safety margin for the active ingredients. A margin of safety is a calculation that takes the no observed adverse effect level (NOAEL) derived from animal data and estimates a maximum safe level of exposure for humans, the data for which would be derived from data generated in the MUsT. In its objection to the proposed MUsT requirements, the comment did not provide an alternative or other validated and accepted approach available to assess human systemic exposure to the active ingredients (Refs. 4 and 21).
(Comment 26) Another comment stated that if MUsTs are to be executed, field studies of health care facility application frequency would be necessary to determine maximum rates as adequate data do not currently exist. The comment asserted that while these studies could take the form of a direct observational study, other avenues may also be considered, such as the use of automated hand hygiene monitoring data. The comment also stated that this data acquisition approach is not subject to behavioral modification interferences by the observer, or hospital department access restrictions, such as the intensive care and surgery units. The comment asserted that this technology has recently progressed substantially in its sophistication and data reliability.
(Response 26) As was mentioned earlier, FDA is discussing the design and conduct of their MUsT program of studies for the six deferred active ingredients.
(Comment 27) One comment submitted in response to the 2015 Health Care Antiseptic PR stated its support for an industry comment submitted to the September 2014 NDAC meeting, which stated that the FDA proposed a safety testing program for OTC products similar to those required for new molecular entity or new chemical entity (NCE) review. The submission asserted that the active ingredients under the 1994 TFM are not NCEs and should not be subjected to requirements that surpass the requirements of a conventional NDA. The submission stated that, in FDA's proposal for the consumer antiseptic wash TFM, the unsubstantiated justification for additional safety data is stated as “new information regarding the potential risks from systemic absorption and long-term exposure to antiseptic active ingredients” and the fact that exposure may be “higher than previously thought,” which, the submission argued, is not supported by information in the 2013 Consumer Antiseptic Wash PR or in the docket.
(Response 27) The assertion that the standards being proposed “surpass the requirements of a conventional NDA” is incorrect. As an example, the MUsT has been required of topical NDA products approved since the early 1990s. Also, a MUsT is often necessary to assess absorption when a topical NDA product is reformulated. Whereas, for the health care antiseptic products under consideration in this rulemaking, once an active ingredient is determined to be GRASE for a particular indication, although in vitro testing would be required under the current framework, no further in vivo studies, including a MUsT, would be required unless in vitro testing suggests that substantially greater absorption may occur with a particular formulation.
3. Carcinogenicity Studies
(Comment 28) Several comments asked FDA to reconsider the requirements for carcinogenicity studies, asserting that a good quality systemic carcinogenicity data set exists, along with in vitro genetic toxicology studies, for the majority of the active ingredients. The comments stated that it is unclear why FDA is requesting additional carcinogenicity studies for these ingredients. The comments also asserted that FDA should justify the requirement for additional carcinogenicity studies by the dermal route of exposure when a carcinogenicity study by the oral route exists because it is highly unlikely that systemic exposure would be higher from the dermal route of exposure than that resulting from the oral route of exposure. One comment requested that FDA focus on the “health effects to be addressed in the safety assessment” rather than establishing “studies to be performed.” Another comment stated that if inhalation carcinogenicity data are available, that such data may be used for worst-case exposure scenarios.
(Response 28) The FDA is requesting dermal carcinogenicity assessment for these topically applied ingredients because the dose that the skin is exposed to following topical exposure can be much higher than the skin dose resulting from systemic exposure (81 FR 61106 at 61123). FDA does not consider in vitro genetic toxicology studies to be a substitute for in vivo carcinogenicity studies. In addition, systemic exposure to the parent drug and metabolites can differ significantly in topically applied products, compared to orally administered products because the skin has its own metabolic capability (81 FR 61106 at 61123). Furthermore, the first-pass metabolism, which is available following oral exposure, is bypassed in the topical route of administration (81 FR 61106 at 61123) (Ref. 22). Dermal carcinogenicity studies, therefore, are not used solely to assess the effect of a drug on the skin tissue, but rather to evaluate the effect of topical exposure to all tissues of the treated animals.
4. Hormonal Effects
(Comment 29) One comment agreed with the Agency that any toxicological risk assessment should consider whether, under conditions of use, an ingredient could cause adverse effects as a result of its ability to interfere with endocrine homeostasis. The comment also agreed with the Agency's statement that general and reproductive toxicology studies are generally adequate to identify potential hormonal effects. The comment urged FDA to take a flexible approach to measuring hormonal effects, and stated that any potential for hormonal effects can be addressed by the interpretation of repeat-dose or developmental and reproductive toxicity testing (DART) data. Specifically, the comment stated that FDA should emphasize that a repeat-dose DART study will provide the point of departure (e.g., NOAEL, Benchmark Dose Lower Bound of 10) for an ingredient that acts by an endocrine mode of action.
(Response 29) We agree that data for hormonal effects can be gleaned from Start Printed Page 60495previously conducted studies (chronic toxicity, DART, and multigenerational studies). As stated in the 2015 Health Care Antiseptic PR, data obtained from general nonclinical toxicity studies and reproductive/developmental studies, such as the repeat-dose toxicity, DART and carcinogenicity, are generally sufficient to identify potential hormonal effects in the developing offspring. We also stated that, if no signals are obtained from these studies, assuming the studies covered all the life stages (i.e., pregnancy, infancy, adolescence), then no further assessment of drug-induced hormonal effects are needed (80 FR 25166 at 25182 to 25183). However, if a positive response is seen in any of these animal studies that requires further investigation, additional studies, such as mechanistic studies, may be needed (Refs. 23, 24, and 25). In terms of the methodology used for the risk assessment of drug products, FDA does not follow the theoretical point of departure approach for assessing toxicological endpoints such as endocrine activity for drug products. Rather, FDA relies on the traditional NOAEL to identify a dose-response relationship in conducting its risk assessment (Refs. 26 and 27).
5. Resistance
(Comment 30) Numerous comments on the issue of bacterial resistance were submitted in response to the 2015 Health Care Antiseptic PR. In general, the comments disagreed on whether antiseptics pose a public health risk from bacterial resistance. Some comments argued that the pervasive use of health care antiseptics poses an unacceptable risk for the development of resistance and that such products should be banned. Other comments argued that antiseptics do not pose such risks and criticized the data on which they believe FDA based its concerns.
Specifically, several comments dismissed the in vitro data cited by FDA in the 2015 Health Care Antiseptic PR as not reflecting real-life conditions. The comments recommended that the most useful assessment of the risk of biocide resistance and cross-resistance to antibiotics are in situ studies, studies of clinical and environmental strains, or biomonitoring studies. Some comments asserted that studies of this type have reinforced the evidence that resistance and cross-resistance associated with antiseptics is a laboratory phenomenon observed only when tests are conducted under unrealistic conditions. One comment stated that there is little credible evidence that antiseptic products play any role in antibiotic resistance in human disease. The comment stated that, while some in vitro lab studies have been successful in forcing expression of resistance in some bacteria to antiseptic active ingredients, real world data from community studies using actual product formulations show no correlation between the use of such products and antibiotic resistance. The comment stated that further evidence of real world data showing no antimicrobial resistance development after the continued use of consumer products containing antimicrobial active compounds can be extracted from oral care clinical studies, which provide in vivo data, under well-controlled conditions, on exposure to antimicrobial-containing formulations over prolonged periods of time (e.g., 6 months to 5 years). Another comment cited the conclusions of an International Conference on Antimicrobial Research held in 2012 on a possible connection between biocide (antiseptic or disinfectant) resistance and antibiotic resistance to support the point that there is no correlation between antiseptic use and antibiotic resistance.
(Response 30) As stated in the 2015 Health Care Antiseptic PR, we continue to believe that the development of bacteria that are resistant to antibiotics is an important public health issue, and additional data may tell us whether use of antiseptics in health care settings may contribute to the selection of bacteria that are less susceptible to both antiseptics and antibiotics (80 FR 25166 at 25183). Thus, we have conducted ingredient-specific reviews of the literature pertaining to antiseptic resistance and antibiotic cross-resistance, and determined that additional studies to assess the development of cross-resistance to antibiotics are needed for three of the deferred active ingredients—benzalkonium chloride, benzethonium chloride, and chloroxylenol. In the case of ethyl alcohol and isopropyl alcohol, sufficient data has been provided to assess the risk of antiseptic resistance and antibiotic cross-resistance.
Laboratory studies have identified and characterized bacterial resistance mechanisms that confer a reduced susceptibility to antiseptics and, in some cases, antibiotics. Specifically, these data suggest that resistance development in the laboratory is very common for some active ingredients, such as benzethonium and benzalkonium chloride (Refs. 28, 29, 30, 31, and 32), and chloroxylenol (Refs. 33, 34, 35, 36, 37, and 38). In contrast, resistance to other active ingredients, such as povidone-iodine (Refs. 39, 40, and 41) occurs infrequently in the laboratory setting. We acknowledge that observations made in the laboratory setting are not necessarily replicated in the real world setting. Therefore, we assessed additional studies performed in the clinical setting.
Studies performed using clinical isolates found strong evidence of antiseptic resistance to benzethonium and benzalkonium chloride (Refs. 42, 43, 44, 45, 46, 47, 48, 49, and 50). Antiseptic resistance genes qacA/B (Ref. 47) and qacE (Ref. 47) were identified and in 83 percent and 73 percent of the isolates tested, respectively, correlated with reduced susceptibility to benzalkonium and benzethonium chloride. In contrast, two studies published by Kawamura-Sato et al. (Refs. 51 and 52) found the MIC of benzalkonium chloride for 283 clinical isolates to be well within in-use concentration.
Only one clinical study could be found assessing resistance to chloroxylenol. Khor et al. (Ref. 53) collected samples from disinfectant solutions in hospitals. Of the chloroxylenol solutions tested, 42 percent had bacterial contamination. Isolation of these bacteria demonstrated that 81 percent were resistant to chloroxylenol, suggesting that these organisms have adapted to survival at concentrations which are usually bactericidal. Clinical studies assessing bacterial resistance to povidone-iodine were primarily negative (Refs. 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, and 64). Only one study, by Mycock et al. (Ref. 65), demonstrated resistance to povidone-iodine using clinical isolates, yet this study could not be repeated (Ref. 66). We believe that there is sufficient information to determine that exposure to povidone-iodine does not lead to the development of bacterial resistance, but additional data is necessary to assess this issue with regards to chloroxylenol.
Other studies examined a possible correlation between antiseptic and antibiotic resistance (Refs. 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 52, 53, 54, 55, 67, 68, 69, 70, 71, and 72). Comparisons suggest that alterations in the mean susceptibility of Staphylococcus aureus to antimicrobial biocides occurred between 1989 and 2000, but these changes were mirrored in both methicillin resistant and susceptible Staphylococcus aureus, suggesting that methicillin resistance has little to do with these changes (Ref. 72). In Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, several correlations (both positive and negative) between antibiotics and antimicrobial biocides Start Printed Page 60496were found (Refs. 52, 54, 56, 67, 70, and 72). From the analyses of these clinical isolates, it is very difficult to support a hypothesis that increased biocide resistance is a cause of increased antibiotic resistance in these species.
In general, studies have not clearly demonstrated an impact of antiseptic bacterial resistance mechanisms in the clinical setting. However, the available studies have limitations. As we noted in the 2015 Health Care Antiseptic PR, studies in a clinical setting that we evaluated were limited by the small numbers and types of organisms, the brief time periods, and the locations examined. Bacteria expressing resistance mechanisms with a decreased susceptibility to antiseptics and some antibiotics have been isolated from a variety of natural settings (Refs. 73 and 74). Although the prevalence of antiseptic tolerant subpopulations in natural microbial populations is currently low, overuse of antiseptic active ingredients has the potential to select for resistant microorganisms.
In sum, adequate data do not exist currently to determine whether the development of bacterial antiseptic resistance could also select for antibiotic resistant bacteria or how significant this selective pressure would be relative to the overuse of antibiotics, an important driver for antibiotic resistance. Moreover, the possible correlation between antiseptic and antibiotic resistance is not the only concern. Reduced antiseptic susceptibility may allow the persistence of organisms in the presence of low-level residues and contribute to the survival of antibiotic resistant organisms. Data are not currently available to assess the magnitude of this risk.
(Comment 31) The comments also disagreed on the data needed to assess the risk of the development of resistance. One comment disagreed with the proposed testing described in the 2015 Health Care Antiseptic PR, arguing that there are no standard laboratory methods for evaluating the development of antimicrobial resistance. With regard to the recommendation for mechanism studies, they believed that it is unlikely that this kind of information can be developed for all active ingredients, particularly given that the mechanism(s) of action may be concentration dependent and combination/formulation effects may be highly relevant. The comments also believed that data characterizing the potential for transferring a resistance determinant to other bacteria is also an unrealistic requirement for a GRAS determination.
Conversely, one comment recommended that antimicrobial resistance be addressed first through in vitro MIC determinations. The comment stated that, if an organism is shown to develop resistance rapidly, FDA should consider this information in its evaluation. The commenter believed that this test of the potential for the development of resistance is important because health care compliance with recommended use of health care antiseptic wash products is variable and products that result in the rapid development of antimicrobial resistance would pose a public health risk. The comment also asserted that GRAS/GRAE ingredients should pose little in the way of a resistance risk.
(Response 31) In the 2015 Health Care Antiseptic PR, we described the data needed to help establish a better understanding of the interactions between antiseptic active ingredients in health care antiseptic products and bacterial resistance mechanisms and the data needed to provide the information necessary to perform an adequate risk assessment for these health care product uses. We suggested a tiered approach as an efficient means of developing data to address this resistance issue—beginning with laboratory studies aimed at evaluating the impact of exposure to nonlethal amounts of antiseptic active ingredients on antiseptic and antibiotic bacterial susceptibilities, along with additional data, if necessary, to help assess the likelihood that changes in susceptibility observed in the preliminary studies would occur in the health care setting (80 FR 25166 at 25183 to 25184).
As we explained in the 2015 Health Care Antiseptic PR, we recognize that the science of evaluating the potential of compounds to cause bacterial resistance is evolving and acknowledged the possibility that alternative data may be identified as an appropriate substitute for evaluating resistance (80 FR 25166 at 25180). We also explained that we are aware that there are no standard protocols for these studies, but there are numerous publications in the literature of studies of this type that could provide guidance on the study design (Refs. 75, 76, and 77).
As explained in this document, we have deferred from this rulemaking six of the active ingredients used in health care antiseptic products, and we are discussing proposed protocols for the safety and effectiveness studies (Refs. 10, 11, 12, 13, 14, and 15). For those active ingredients for which resistance testing is required—chloroxylenol, benzethonium chloride, and benzalkonium chloride—we have advised manufacturers, as an initial step, to conduct an active ingredient-specific literature review related to antiseptic resistance and antibiotic cross-resistance to assess the active ingredient's effect on development of cross-resistance to antiseptics and antibiotics in the health care setting, and to submit as much information and data as can be provided. If the literature review results show evidence of antiseptic or antibiotic resistance, additional studies may be necessary, consistent with the recommendations outlined in the 2015 Health Care Antiseptic PR (80 FR 25166 at 25183 to 25184), to help assess the impact of the active ingredient on antiseptic and antibiotic susceptibilities. If, however, the literature review provides no evidence that the active ingredient affects antiseptic or antibiotic susceptibility, then it is likely that no further studies to address development of resistance will be needed to support a GRAS determination.
6. Other Safety Issues
(Comment 32) One comment also stated that FDA's evaluation of risks associated with the extensive use of health care antiseptic soaps by health care workers should include the data from the Nurses' Health Studies (NHS), which are a series of long-term studies of health outcomes in several large cohorts of nurses. The comment asserted that these studies did not show any evidence that the use of topical health care antiseptics leads to adverse health outcomes in nurses. The comment concedes that the studies were not designed to evaluate risks associated with the use of antiseptic soaps, but still believes these studies are adequate to detect clinically-relevant health outcomes, including those associated with endocrine effects, that might arise from the use of antiseptic soaps.
The comment also noted that the FDA's Safety Information and Adverse Event Reporting Program, MedWatch, did not have any safety-related reports on the health care antiseptic products identified in the 2015 Health Care Antiseptic PR. In addition, the comment stated that FDA has not issued any safety alerts related to antiseptic skin products.
(Response 32) FDA searched the NHS website cited in the comment, www.channing.harvard.edu/nhs/,, and there did not appear to be any studies listed that specifically evaluated the health outcomes of nurses after using health care antiseptics. As the comment noted, the NHS studies were not designed to evaluate risks associated with the use of antiseptic soaps. In addition, in order to effectively evaluate the safety of an active ingredient or Start Printed Page 60497drug, FDA uses data in which a control group is included in the study to compare to the treatment groups. A prospective NHS study evaluating the effect of exposure to the active ingredients in health care antiseptics would require a control group in which there is no exposure to health care antiseptic active ingredients. However, because all nurses in health care environments in which NHS studies have been conducted have to adhere to a universal hand washing protocol using antiseptic active ingredients, it is not possible to include a control group with no exposure to healthcare antiseptics in a NHS study.
We also note that the safety signals FDA uses in making a GRAS determination, such as developmental and reproductive toxicity, carcinogenicity, or hormonal effects, would not likely be reported by consumers or health care professionals to MedWatch. Thus, the lack of MedWatch safety-related reports does not eliminate the need for the safety data outlined in the 2015 Health Care Antiseptic PR.
(Comment 33) One comment stated that, for FDA to fully assess the safety of the health care topical antiseptic active ingredients, it must consider the impact of exposure on groups that may be particularly sensitive to exposure, including pregnant women, children, and the elderly, particularly with regards to chronic or highly sensitive (e.g., newborn infant) exposure.
The comment also proposed that in classifying an ingredient as GRAS/GRAE, FDA should expand the health impacts (e.g., impact on the microbiome) and should consider “clinically-relevant” effectiveness (e.g., reduction of bacteria typically found in health care settings). The comment added that the final rule should incorporate safety standards to protect populations, outside of health care personnel, that could experience increased adverse events upon exposure to antiseptic products. The comment contended that the effect of antiseptic active ingredients on the microbiome should be more thoroughly considered in the final monograph to incorporate the effects into the benefit-to-risk calculation.
The comment also asserted that data used in the safety evaluation of these ingredients should include metabolic parameters of disease states of individuals who would be chronically exposed to health care antiseptics in animal pharmacokinetic absorption, distribution, metabolism, and excretion (ADME) models.
(Response 33) We agree that the impact of exposure to sensitive populations should be considered. Our paradigm of safety evaluation, which includes a battery of safety studies (ADME, MUsT, carcinogenicity, DART, and hormonal effects), can be used to establish a safety margin for potential safety signals in all populations, including sensitive ones.
Currently, the effect of health care antiseptic active ingredients on the microbiome have not been included as a safety signal in classifying an active ingredient as GRAS or non-GRAS. FDA will continue to monitor emerging technologies that can help address safety signals for all of the products that it regulates, including products under the OTC topical antiseptic monograph.
In addition, because there are many disease states which health care professionals or patients could have, it is not feasible to develop metabolic parameters for individual disease states in conducting the GRAS determinations of the active ingredients used in health care antiseptic products. Nor could one prospectively identify which specific metabolic parameters should be tracked, or if there were defined levels of changes in each parameter that would be of concern.
(Comment 34) Another comment stated that FDA needs to address the impact of inactive ingredients and final formulations on the safety assessments of health care antiseptic products.
(Response 34) Testing requirements for the final product formulations, which would require exposure to both active and inactive ingredients, are not addressed in this final rule because none of the active ingredients that are the subject of this final rule are considered GRAS/GRAE for use in health care antiseptic products, given the lack of sufficient effectiveness and safety data submitted for these ingredients. The testing requirements for final formulations of products containing the six deferred active ingredients will be addressed, if applicable, after a decision is made regarding the monograph status of those ingredients.
(Comment 35) One comment indicated that the cost of conducting safety studies is expensive and asserted that the testing requirements run counter to the spirit of the OTC monograph. The comment proposed that the safety studies, should therefore, be conducted by academic and National Institutes of Health (NIH) investigators.
(Response 35) The monograph process is public in nature and studies may be conducted by any interested parties, including academics and NIH investigators. FDA is willing to review all relevant available data in order to reach a final determination of safety and effectiveness. Ultimately, manufacturers are responsible for the safety and effectiveness of the drug products they market.
(Comment 36) One comment contended that NDA products, such as Avagard (1 percent chlorhexidine gluconate, 62 percent ethyl alcohol) should be subject to the safety standards proposed in the 2015 Health Care Antiseptic PR.
(Response 36) FDA regulates NDA products under a different regulatory pathway than the OTC drug monograph products, such as the OTC health care antiseptics that are the subject of this rulemaking. We consider safety criteria for both monograph and NDA products. The review of an individual product under an NDA may warrant a different assessment than a group of active ingredients used in a range of products.
F. Comments on the Preliminary Regulatory Impact Analysis and FDA Response
(Comment 37) Several comments raised issues concerning the preliminary regulatory impact analysis and the Agency's assessment of the net benefit of the rulemaking.
(Response 37) Our response is provided in the full discussion of economic impacts, available in the docket for this rulemaking (Docket No. FDA-2015-N-0101, (Ref. 78), https://www.regulations.gov) and at https://www.fda.gov/AboutFDA/ReportsManualsForms/Reports/EconomicAnalyses/default.htm.
VI. Ingredients Not Generally Recognized as Safe and Effective
No additional safety or effectiveness data have been submitted to support a GRAS/GRAE determination for the non-deferred health care antiseptic active ingredients described in this rule. Thus, the following active ingredients are not GRAS/GRAE for use as a health care antiseptic:
- Chlorhexidine gluconate
- Cloflucarban
- Fluorosalan
- Hexachlorophene
- Hexylresorcinol
- Iodophors (Iodine-containing ingredients)
○ Iodine complex (ammonium ether sulfate and polyoxyethylene sorbitan monolaurate)
○ Iodine complex (phosphate ester of alkylaryloxy polyethylene glycol)
○ Iodine tincture USP
○ Iodine topical solution USP
○ Nonylphenoxypoly (ethyleneoxy) ethanoliodineStart Printed Page 60498
○ Poloxamer—iodine complex
○ Undecoylium chloride iodine complex
- Mercufenol chloride
- Methylbenzethonium chloride
- Phenol
- Secondary amyltricresols
- Sodium oxychlorosene
- Tribromsalan
- Triclocarban
- Triclosan
- Triple dye
- Combination of calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative
- Combination of mercufenol chloride and secondary amyltricresols in 50 percent alcohol
Accordingly, OTC health care antiseptic drug products containing these active ingredients will require approval under an NDA or ANDA prior to marketing.
VII. Compliance Date
In the 2015 Health Care Antiseptic PR, we recognized, based on the scope of products subject to this final rule, that manufacturers would need time to comply with this final rule. Thus, as proposed in the 2015 Health Care Antiseptic PR (80 FR 25166 at 25195), this final rule will be effective 1 year after the date of the final rule's publication in the Federal Register. On or after that date, any OTC health care antiseptic drug products containing an ingredient that we have found in this final rule to be not GRAS/GRAE cannot be introduced or delivered for introduction into interstate commerce unless it is the subject of an approved NDA or ANDA.
VIII. Summary of Regulatory Impact Analysis
The summary analysis of benefits and costs included in this final rule is drawn from the detailed Regulatory Impact Analysis that is available at https://www.regulations.gov, Docket No. FDA-2015-N-0101, (Ref. 78).
A. Introduction
We have examined the impacts of the final rule under Executive Order 12866, Executive Order 13563, Executive Order 13771, the Regulatory Flexibility Act (5 U.S.C. 601-612), and the Unfunded Mandates Reform Act of 1995 (Pub. L. 104-4). Executive Orders 12866 and 13563 direct us to assess all costs and benefits of available regulatory alternatives and, when regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety, and other advantages; distributive impacts; and equity). Executive Order 13771 requires that the costs associated with significant new regulations “shall, to the extent permitted by law, be offset by the elimination of existing costs associated with at least two prior regulations.” We believe that this final rule is a significant regulatory action as defined by Executive Order 12866. This final rule is considered an Executive Order 13771 regulatory action.
The Regulatory Flexibility Act requires us to analyze regulatory options that would minimize any significant impact of a rule on small entities. Because we estimate that only four small businesses will be adversely affected by the final rule, we certify that the final rule will not have a significant economic impact on a substantial number of small entities.
The Unfunded Mandates Reform Act of 1995 (Section 202(a)) requires us to prepare a written statement, which includes an assessment of anticipated costs and benefits, before proposing “any rule that includes any Federal mandate that may result in the expenditure by State, local, and tribal governments, in the aggregate, or by the private sector, of $100,000,000 or more (adjusted annually for inflation) in any one year.” The current threshold after adjustment for inflation is $148 million, using the most current (2016) Implicit Price Deflator for the Gross Domestic Product. This final rule would not result in an expenditure in any year that meets or exceeds this amount
B. Summary of Costs and Benefits
As discussed in the preamble of this final rule, this rule establishes that 24 eligible active ingredients are not generally recognized as safe and effective for use in OTC health care antiseptics. However, data from the FDA drug product registration database suggest that only one of these 24 ingredients is found in OTC health care antiseptic products currently marketed pursuant to the TFM: Triclosan. Regulatory action is being deferred on six active ingredients that were addressed in the health care antiseptic proposed rule: Benzalkonium chloride, benzethonium chloride, chloroxylenol, ethyl alcohol, isopropyl alcohol, and povidone-iodine. This final rule also addresses the eligibility of three active ingredients—alcohol (ethyl alcohol, see section V.C.3), benzethonium chloride, and chlorhexidine gluconate—and finds that these three active ingredients are ineligible for evaluation under the OTC Drug Review for certain health care antiseptic uses (see section IV.D.1, table 3). To our knowledge, there is only one ineligible product currently on the market, an alcohol-containing surgical hand scrub, which is affected by this rule.
Benefits are quantified as the volume reduction in exposure to triclosan found in health care antiseptic products affected by the rule, but these benefits are not monetized. Annual benefits are estimated to be a reduction in exposure of 88,000 kg of triclosan per year.
Costs are calculated as the one-time costs associated with reformulating health care antiseptic products containing the active ingredient triclosan and relabeling reformulated products, plus the lost producer surplus (measured as lost revenues) due to removing one alcohol surgical hand scrub from the market. We believe that the alcohol-containing surgical hand scrub that is affected by this rule is likely to be removed from the market. We categorize the associated loss of sales revenue as a transfer from one manufacturer to another and not a cost, because we assume that the supply of other, highly substitutable, products is highly elastic.
Annualizing the one-time costs over a 10-year period, we estimate total annualized costs to range from $1.1 to $4.1 million at a 3 percent discount rate, and from $1.2 to $4.7 million at a 7 percent discount rate. The present value of total costs ranges from $9.0 to $34.6 million at a 3 percent discount rate, and from $8.7 to $29.6 million at a 7 percent discount rate.
In this final rule, small entities will bear costs to the extent that they must reformulate and re-label any health care antiseptic containing triclosan that they produce. The average cost to small firms of implementing the requirements of this final rule is estimated to be $213,176 per firm. The costs of the changes, along with the small number of firms affected, implies that this burden would not be significant, so we certify that this final rule will not have a significant economic impact on a substantial number of small entities. This analysis, together with other relevant sections of this document, serves as the Regulatory Flexibility Analysis, as required under the Regulatory Flexibility Act.
We have developed a comprehensive Economic Analysis of Impacts that assesses the impacts of the final rule. The full analysis of economic impacts is available in docket FDA-2015-N-0101 (Ref. 78) and at https://www.fda.gov/AboutFDA/ReportsManualsForms/Reports/EconomicAnalyses/default.htm.
Start Printed Page 60499Table 5—Executive Order 13771 Summary Table
[In $ millions 2016 dollars, over an infinite time horizon]
Primary (7%) Lower bound (7%) Upper bound (7%) Present value of costs $17.19 $8.68 $29.47 Present Value of Cost Savings Present Value of Net Costs 17.19 8.68 29.47 Annualized Costs 1.20 0.61 2.06 Annualized Cost Savings Annualized Net Costs 1.20 0.61 2.06 IX. Paperwork Reduction Act of 1995
This final rule contains no collection of information. Therefore, clearance by OMB under the Paperwork Reduction Act of 1995 is not required.
X. Analysis of Environmental Impact
We have determined under 21 CFR 25.31(a) that this action is of a type that does not individually or cumulatively have a significant effect on the human environment. Therefore, neither an environmental assessment nor an environmental impact statement is required.
XI. Federalism
We have analyzed this final rule in accordance with the principles set forth in Executive Order 13132. Section 4(a) of the Executive order requires agencies to “construe . . . a Federal statute to preempt State law only where the statute contains an express preemption provision or there is some other clear evidence that the Congress intended preemption of State law, or where the exercise of State authority conflicts with the exercise of Federal authority under the Federal statute.” The sole statutory provision giving preemptive effect to the final rule is section 751 of the FD&C Act (21 U.S.C. 379r). We have complied with all of the applicable requirements under the Executive order and have determined that the preemptive effects Start Printed Page 60500of this rule are consistent with Executive Order 13132.
XII. References
The following references are on display at the office of the Dockets Management Staff (see ADDRESSES) and are available for viewing by interested persons between 9 a.m. and 4 p.m., Monday through Friday; they are also available electronically at https://www.regulations.gov. FDA has verified all website addresses, as of the date of this document publishes in the Federal Register, but websites are subject to change over time.
1. Transcript of the January 22, 1997, Joint Meeting of the Nonprescription Drugs and Anti-Infective Drugs Advisory Committees, OTC Vol. 230002. Available at https://www.regulations.gov/document?D=FDA-2015-N-0101-0008.
2. Comment submitted in Docket No. FDA-1975-N-0012-0081. Available at https://www.regulations.gov/document?D=FDA-1975-N-0012-0081.
3. Transcript of the March 23, 2005, Nonprescription Drugs Advisory Committee. Available at http://www.fda.gov/ohrms/dockets/ac/05/transcripts/2005-4098T1.htm.
4. Transcript of the September 3, 2014, Meeting of the Nonprescription Drugs Advisory Committee 2014. Available at https://wayback.archive-it.org/7993/20170404152741/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/NonprescriptionDrugsAdvisoryCommittee/UCM421121.pdf.
5. Part 130-New Drugs, Procedures for Classification of Over-the-Counter Drugs. Available at http://www.ecfr.gov/cgi-bin/text-idx?SID=34d4a9d52dbac32a09a126a1dd44ef47&mc=true&node=se21.5.330_110&rgn=div8.
6. Tuuli, M.G., et al., A Randomized Trial Comparing Skin Antiseptic Agents at Cesarean Delivery, New England Journal of Medicine, 374(7): p. 647-55, 2016. Available at https://www.ncbi.nlm.nih.gov/pubmed/26844840.
7. Centers for Disease Control and Prevention, Guideline for Hand Hygiene in Health-Care Settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force, Morbidity and Mortality Weekly Report, 51(RR-16): p. 1-45, 2002. Available at https://www.ncbi.nlm.nih.gov/pubmed/12418624.
8. Mangram, A.J., et al., Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention Hospital Infection Control Practices Advisory Committee, American Journal of Infection Control, 27(2): p. 97-132, 1999. Available at http://www.ncbi.nlm.nih.gov/pubmed/10196487.
9. Larson, E., Innovations in Health Care: Antisepsis as a Case Study, American Journal of Public Health, 79(1): p. 92-99, 1989. Available at http://www.ncbi.nlm.nih.gov/pubmed/2642372.
10. FDA Deferral Letter for Benzalkonium Chloride in Health Care Antiseptics on January 19, 2017. Available at https://www.regulations.gov/document?D=FDA-2015-N-0101-1321.
11. FDA Deferral Letter for Benzethonium Chloride in Health Care Antiseptics on January 19, 2017. Available at https://www.regulations.gov/document?D=FDA-2015-N-0101-1322.
12. FDA Deferral Letter for Chloroxylenol in Health Care Antiseptics on January 19, 2017. Available at https://www.regulations.gov/document?D=FDA-2015-N-0101-1323.
13. FDA Deferral Letter for Ethyl Alcohol in Health Care Antiseptics on January 19, 2017. Available at https://www.regulations.gov/document?D=FDA-2015-N-0101-1324.
14. FDA Deferral Letter for Isopropyl Alcohol in Health Care Antiseptics on January 19, 2017. Available at https://www.regulations.gov/document?D=FDA-2015-N-0101-1325.
15. FDA Deferral Letter for Povidone Iodine in Health Care Antiseptics on January 19, 2017. Available at https://www.regulations.gov/document?D=FDA-2015-N-0101-1326.
16. Wheelock, S.M. and S. Lookinland, Effect of surgical hand scrub time on subsequent bacterial growth, Association of periOperative Registered Nurses Journal, 65(6): p. 1087-92; 94-8, 1997. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=9187454.
17. Rotter, M.L., et al., Population kinetics of the skin flora on gloved hands following surgical hand disinfection with 3 propanol-based hand rubs: a prospective, randomized, double-blind trial, Infection Control Hospital Epidemiology, 28(3): p. 346-50, 2007. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=17326028.
18. Beldame, J., et al., Surgical glove bacterial contamination and perforation during total hip arthroplasty implantation: when gloves should be changed, Orthopaedics & Traumatology: Surgery & Research, 98(4): p. 432-40, 2012. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=22578871.
19. Summary Minutes of the September 3, 2014, Nonprescription Drugs Advisory Committee Meeting. Available at https://wayback.archive-it.org/7993/20170404152740/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/NonprescriptionDrugsAdvisoryCommittee/UCM421120.pdf.
20. Dunnick, J.K. and J.R. Hailey, Phenolphthalein Exposure Causes Multiple Carcinogenic Effects in Experimental Model Systems, Cancer Research, 56(21): p. 4922-26, 1996. Available at https://www.ncbi.nlm.nih.gov/pubmed/8895745.
21. Briefing Material for the September 2014, Meeting of the Nonprescription Drugs Advisory Committee. Available at https://wayback.archive-it.org/7993/20170404152730/https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/NonprescriptionDrugsAdvisoryCommittee/ucm410287.htm.
22. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, Guideline for Industry. The Need for Carcinogenicity Studies of Pharmaceuticals. Available at http://www.fda.gov/downloads/drugs/.../guidances/ucm074911.pdf.
23. Food and Drug Administration, Draft Guidance for Industry. Endocrine Disruption Potential of Drugs: Nonclinical Evaluation. Available at https://www.federalregister.gov/documents/2013/09/20/2013-22864/draft-guidance-for-industry-on-endocrine-disruption-potential-of-drugs-nonclinical-evaluation.
24. Food and Drug Administration, Redbook 2000: IV.C.9.a: Reproduction and Developmental Toxicity Studies: Guidelines for Reproduction Studies. Available at http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/IngredientsAdditivesGRASPackaging/ucm2006826.htm.
25. United States Environmental Protection Agency, Guidelines for reproductive toxicity risk assessment. Available at http://www2.epa.gov/sites/production/files/2014-11/documents/guidelines_repro_toxicity.pdf.
26. Food and Drug Administration, Guidance for Industry. M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals. Available at www.fda.gov/downloads/drugs/guidances/ucm073246.pdf.
27. Food and Drug Administration, Guidance for Industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers Available at www.fda.gov/downloads/drugs/guidances/ucm078932.pdf.
28. Bearden, D.T., et al., Comparative In Vitro Activities of Topical Wound Care Products Against Community-Associated Methicillin-Resistant Staphylococcus aureus, Journal of Antimicrobial Chemotherapy, 62(4): p. 769-72, 2008. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=18593725.
29. Czerwinski, S.E., et al., Novel Water-Based Antiseptic Lotion Demonstrates Rapid, Broad-Spectrum Kill Compared with Alcohol Antiseptic, Journal of Infection and Public Health, 7(3): p. 199-204, 2014. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=24810728.
30. Nagai, I. and H. Ogase, Absence of Role for Plasmids in Resistance to Multiple Disinfectants in Three Strains of Bacteria, Journal of Hospital Infection, 15(2): p. 149-55, 1990. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=1969437.
31. Geraldo, I.M., et al., Rapid Antibacterial Activity of 2 Novel Hand Soaps: Start Printed Page 60501Evaluation of the Risk of Development of Bacterial Resistance to the Antibacterial Agents, Infection Control and Hospital Epidemiology, 29(8): p. 736-41, 2008. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=18616390.
32. Sakagami, Y. and K. Kajimura, Bactericidal Activities of Disinfectants against Vancomycin-Resistant Enterococci, Journal of Hospital Infection, 50(2): p. 140-44, 2002. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=11846542.
33. Haley, C.E., et al., Bactericidal Activity of Antiseptics Against Methicillin-Resistant Staphylococcus aureus, Journal of Clinical Microbiology, 21(6): p. 991-92, 1985. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=4008627.
34. Johnson, S.A., et al., Comparative Susceptibility of Resident and Transient Hand Bacteria to Para-chloro-meta-xylenol and Triclosan, Journal of Applied Microbiology, 93(2): p. 336-44, 2002. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=12147083.
35. Kolawole, D.O., Resistance Mechanisms of Mucoid-Grown Staphylococcus aureus to the Antibacterial Action of Some Disinfectants and Antiseptics, FEMS Microbiology Letters, 25(2-3): p. 205-09, 1984. Available at http://www.sciencedirect.com/science/article/pii/0378109784901198.
36. Sivaji, Y. and A. Mandal, Antibiotic Resistance and Serotypic Variations in Escherichia coli Induced by Disinfectants, Indian Journal of Medical Research, 86(2): p. 165-72, 1987. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=3323039.
37. Sivaji, Y., et al., Disinfectant Induced Changes in the Antibiotic Sensitivity and Phage Typing Pattern in Staphylococcus aureus, Journal of Hospital Infection, 7(3): p. 236-43, 1986. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=2873169.
38. Young, R., et al., Comparative In Vitro Efficacy of Antimicrobial Shampoos: A Pilot Study, Veterinary Dermatology, 23(1): p. 36-40, 2012. Available at https://www.ncbi.nlm.nih.gov/pubmed/21777309.
39. Block, C., et al., Evaluation of Chlorhexidine and Povidone Iodine Activity Against Methicillin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus faecalis Using a Surface Test, Journal of Hospital Infection, 46(2): p. 147-52, 2000. Available at https://www.ncbi.nlm.nih.gov/pubmed/11049709.
40. Kunisada, T., et al., Investigation on the Efficacy of Povidone-Iodine Against Antiseptic-Resistant Species, Dermatology, 195 Suppl 2(1): p. 14-8, 1997. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=9403250.
41. Lacey, R.W., et al., Antiseptic Resistance in Methicillin-Resistant Staphylococcus aureus, The Lancet, 326(8467): p. 1307-08, 1985. Available at https://www.ncbi.nlm.nih.gov/pubmed/2866372.
42. Liu, Q., et al., Sensitivities to Biocides and Distribution of Biocide Resistance Genes in Quaternary Ammonium Compound Tolerant Staphylococcus aureus Isolated in a Teaching Hospital, Scandinavian Journal of Infectious Diseases, 41(6-7): p. 403-09, 2009. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=19353380.
43. Furi, L., et al., Evaluation of Reduced Susceptibility to Quaternary Ammonium Compounds and Bisbiguanides in Clinical Isolates and Laboratory-Generated Mutants of Staphylococcus aureus, Antimicrobial Agents and Chemotherapy, 57(8): p. 3488-97, 2013. Available at http://www.ncbi.nlm.nih.gov/pubmed/23669380.
44. Babaei, M., et al., Extremely High Prevalence of Antiseptic Resistant Quaternary Ammonium Compound E Gene Among Clinical Isolates of Multiple Drug Resistant Acinetobacter baumannii in Malaysia, Annals of Clinical Microbiology and Antimicrobials, 14(1): p. 11, 2015. Available at https://www.ncbi.nlm.nih.gov/pubmed/25858356.
45. Ghasemzadeh-Moghaddam, H., et al., Methicillin-Susceptible and -Resistant Staphylococcus aureus With High-Level Antiseptic and Low-Level Mupirocin Resistance in Malaysia, Microbial Drug Resistance, 20(5): p. 472-77, 2014. Available at https://www.ncbi.nlm.nih.gov/pubmed/24841796.
46. Noguchi, N., et al., Susceptibilities to Antiseptic Agents and Distribution of Antiseptic-Resistance Genes qacA/B and smr of Methicillin-Resistant Staphylococcus aureus isolated in Asia During 1998 and 1999, Journal of Medical Microbiology, 54(1): p. 557-65, 2005. Available at https://www.ncbi.nlm.nih.gov/pubmed/15888465.
47. Shamsudin, M.N., et al., High Prevalence of qacA/B Carriage Among Clinical Isolates of Meticillin-Resistant Staphylococcus aureus in Malaysia, Journal of Hospital Infection, 81(3): p. 206-8, 2012. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=22633074.
48. Weber, D.J. and W.A. Rutala, Use of Germicides in the Home and the Healthcare Setting: Is There a Relationship between Germicide Use and Antibiotic Resistance?, Infection Control and Hospital Epidemiology, 27(10): p. 1107-19, 2006. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=17006819.
49. Rajamohan, G., et al., Biocide-Tolerant Multidrug-Resistant Acinetobacter baumannii Clinical Strains Are Associated with Higher Biofilm Formation, Journal of Hospital Infection, 73(3): p. 287-89, 2009. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=19762119.
50. Akinkunmi, E.O. and A. Lamikanra, Susceptibility of Community Associated Methicillin Resistant Staphylococcus aureus Isolated From Faeces to Antiseptics, Journal of Infection in Developing Countries, 6(4): p. 317-23, 2012. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=22505440.
51. Kawamura-Sato, K., et al., Reduction of Disinfectant Bactericidal Activities in Clinically Isolated Acinetobacter species in the Presence of Organic Material, Journal of Antimicrobial Chemotherapy, 61(3): p. 568-76, 2008. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=18192683.
52. Kawamura-Sato, K., et al., Correlation Between Reduced Susceptibility to Disinfectants and Multidrug Resistance Among Clinical Isolates of Acinetobacter Species, Journal of Antimicrobial Chemotherapy, 65(9): p. 1975-83, 2010. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=20576639.
53. Khor, S.Y. and M. Jegathesan, In-Use testing of Disinfectants in Malaysian Government Hospitals, Medical Journal of Malaysia, 32(1): p. 85-89, 1977. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=609352.
54. Rikimaru, T., et al., Bactericidal Activities of Commonly Used Antiseptics Against Multidrug-Resistant Mycobacterium tuberculosis, Dermatology, 204(Supp): p. 15-20, 2002. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=12011515.
55. Lanker Klossner, B., et al., Nondevelopment of Resistance by Bacteria During Hospital Use of Povidone-Iodine, Dermatology, 195(Supp 2): p. 10-13, 1997. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=9403249.
56. Prince, H.N., et al., Drug Resistance Studies with Topical Antiseptics, Journal of Pharmaceutical Sciences, 67(11): p. 1629-31, 1978. Available at https://www.ncbi.nlm.nih.gov/pubmed/712607.
57. Wiegand, C., et al., Analysis of the Adaptation Capacity of Staphylococcus aureus to Commonly Used Antiseptics by Microplate Laser Nephelometry, Skin Pharmacology and Physiology, 25(6): p. 288-97, 2012. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=22890487.
58. Giacometti, A., et al., Antiseptic Compounds Still Active Against Bacterial Strains Isolated From Surgical Wound Infections Despite Increasing Antibiotic Resistance, European Journal of Clinical Microbiology and Infectious Diseases, 21(7): p. 553-56, 2002. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=12172750.
59. Gocke, D.J., et al., In Vitro Studies of the Killing of Clinical Isolates by Povidone-Start Printed Page 60502Iodine Solutions, Journal of Hospital Infection, 6(Supp A): p. 59-66, 1985. Available at http://www.ncbi.nlm.nih.gov/pubmed/2860177.
60. Yasuda, T., et al., Comparison of Bactericidal Activities of Various Disinfectants Against Methicillin-Sensitive Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus, Postgraduate Medical Journal, 69(Supp 3): p. 66-69, 1993. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=8290461.
61. Gordon, J. and A.R. Mclure, In-Vitro Comparison of Bactericidal Activity of Povidone-Iodine and Chlorhexidine Against Methicillin-Resistant Staphylococcus aureus, Surgical Research Communications, 5(4): p. 313-14, 1989. Available at http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L19121090.
62. Barry, A.L., et al., Lack of Effect of Antibiotic Resistance on Susceptibility of Microorganisms to Chlorhexidine Gluconate or Povidone Iodine, European Journal of Clinical Microbiology and Infectious Diseases, 18(12): p. 920-21, 1999. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=10691210.
63. Rikimaru, T., et al., Efficacy of Common Antiseptics Against Multidrug-Resistant Mycobacterium tuberculosis, International Journal of Tuberculosis and Lung Disease, 6(9): p. 763-70, 2002. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=12234131.
64. Sakuragi, T., et al., Bactericidal Activity of Skin Disinfectants on Methicillin-Resistant Staphylococcus aureus, Anesthesia and Analgesia, 81(3): p. 555-58, 1995. Available at http://www.ncbi.nlm.nih.gov/pubmed/7653822.
65. Sanchez, P., et al., The Biocide Triclosan Selects Stenotrophomonas maltophilia Mutants That Overproduce the SmeDEF Multidrug Efflux Pump, Antimicrobial Agents and Chemotherapy, 49(2): p. 781-82, 2005. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=15673767.
66. Payne, D.N., et al., Antiseptics: A Forgotten Weapon in the Control of Antibiotic Resistant Bacteria in Hospital and Community Settings?, Journal of the Royal Society of Health, 118(1): p. 18-22, 1998. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=9724934.
67. Oggioni, M.R., et al., Significant Differences Characterize the Correlation Coefficients Between Biocide and Antibiotic Susceptibility Profiles in Staphylococcus aureus, Current Pharmaceutical Design, 21(16): p. 2054-57, 2015. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=25760337.
68. Coelho, J.R., et al., The Use of Machine Learning Methodologies to Analyse Antibiotic and Biocide Susceptibility in Staphylococcus aureus, PLoS One, 8(2): p. 1, 2013. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=23431361.
69. Stickler, D.J., et al., Antiseptic and Antibiotic Resistance in Gram-Negative Bacteria Causing Urinary Tract Infection in Spinal Cord Injured Patients, Paraplegia, 19(1): p. 50-58, 1981. Available at https://www.ncbi.nlm.nih.gov/pubmed/7220061.
70. Buffet-Bataillon, S., et al., Effect of Higher Minimum Inhibitory Concentrations of Quaternary Ammonium Compounds in Clinical E. coli Isolates on Antibiotic Susceptibilities and Clinical Outcomes, Journal of Hospital Infection, 79(2): p. 141-46, 2011. Available at http://www.ncbi.nlm.nih.gov/pubmed/21807440.
71. Shi, G.S., et al., Prevalence of Antiseptic-Resistance Genes in Staphylococci Isolated from Orthokeratology Lens and Spectacle Wearers in Hong Kong, Investigative Ophthalmology and Visual Science, 56(5): p. 3069-74, 2015. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=25788652.
72. Lambert, R.J., Comparative Analysis of Antibiotic and Antimicrobial Biocide Susceptibility Data in Clinical Isolates of Methicillin-Sensitive Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa between 1989 and 2000, Journal of Applied Microbiology, 97(4): p. 699-711, 2004. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=15357719.
73. Morrissey, I., et al., Evaluation of Epidemiological Cut-Off Values Indicates That Biocide Resistant Subpopulations Are Uncommon in Natural Isolates of Clinically-Relevant Microorganisms, PLoS One, 9(1): p. 86669, 2014. Available at http://www.ncbi.nlm.nih.gov/pubmed/24466194.
74. Copitch, J.L., et al., Prevalence of Decreased Susceptibility to Triclosan in Salmonella enterica Isolates from Animals and Humans and Association with Multiple Drug Resistance, International Journal of Antimicrobial Agents, 36(3): p. 247-51, 2010. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=20541914.
75. Braoudaki, M. and A.C. Hilton, Adaptive Resistance to Biocides in Salmonella enterica and Escherichia coli O157 and Cross-Resistance to Antimicrobial Agents, Journal of Clinical Microbiology, 42(1): p. 73-78, 2004. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=14715734.
76. Langsrud, S., et al., Cross-Resistance to Antibiotics of Escherichia coli Adapted to Benzalkonium Chloride or Exposed to Stress-Inducers, Journal of Applied Microbiology, 96(1): p. 201-08, 2004. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=14678175.
77. Joynson, J.A., et al., Adaptive Resistance to Benzalkonium Chloride, Amikacin and Tobramycin: The Effect on Susceptibility to Other Antimicrobials, Journal of Applied Microbiology, 93(1): p. 96-107, 2002. Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=12067378.
78. FDA Regulatory Impact Analysis, Safety and Effectiveness for Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Available at http://www.fda.gov/AboutFDA/ReportsManualsForms/Reports/EconomicAnalyses/default.htm.
Start List of SubjectsList of Subjects in 21 CFR Part 310
- Administrative practice and procedure
- Drugs
- Labeling
- Medical devices
- Reporting and recordkeeping requirements
Therefore, under the Federal Food, Drug, and Cosmetic Act and under authority delegated to the Commissioner of Food and Drugs, 21 CFR part 310 is amended as follows:
Start PartPART 310—NEW DRUGS
End Part Start Amendment Part1. The authority citation for part 310 continues to read as follows:
End Amendment Part Start Amendment Part2. Amend § 310.545 as follows:
End Amendment Part Start Amendment Parta. Add reserved paragraphs (a)(27)(v), (vii), and (ix);
End Amendment Part Start Amendment Partb. Add paragraphs (a)(27)(vi), (viii), and (x);
End Amendment Part Start Amendment Partc. In paragraph (d) introductory text, remove “(d)(41)” and in its place add “(42)”; and
End Amendment Part Start Amendment Partd. Add paragraph (d)(42).
End Amendment PartThe additions read as follows:
Start SignatureDrug products containing certain active ingredients offered over-the-counter (OTC) for certain uses.(a) * * *
(27) * * *
(v) [Reserved]
(vi) Health care personnel hand wash drug products. Approved as of December 20, 2018.
Cloflucarban
Fluorosalan
Hexachlorophene
Hexylresorcinol
Iodine complex (ammonium ether sulfate and polyoxyethylene sorbitan monolaurate)
Iodine complex (phosphate ester of alkylaryloxy polyethylene glycol)
Methylbenzethonium chloride
Nonylphenoxypoly (ethyleneoxy) ethanoliodine
Phenol
Poloxamer-iodine complex
Secondary amyltricresols
Sodium oxychlorosene
Tribromsalan
Triclocarban
Triclosan
Undecoylium chloride iodine complex
(vii) [Reserved]
(viii) Surgical hand scrub drug products. Approved as of December 20, 2018.
Start Printed Page 60503Cloflucarban
Fluorosalan
Hexachlorophene
Hexylresorcinol
Iodine complex (ammonium ether sulfate and polyoxyethylene sorbitan monolaurate)
Iodine complex (phosphate ester of alkylaryloxy polyethylene glycol)
Methylbenzethonium chloride
Nonylphenoxypoly (ethyleneoxy) ethanoliodine
Phenol
Poloxamer-iodine complex
Secondary amyltricresols
Sodium oxychlorosene
Tribromsalan
Triclocarban
Triclosan
Undecoylium chloride iodine complex
(ix) [Reserved]
(x) Patient antiseptic skin preparation drug products. Approved as of December 20, 2018.
Cloflucarban
Fluorosalan
Hexachlorophene
Hexylresorcinol
Iodine complex (phosphate ester of alkylaryloxy polyethylene glycol)
Iodine tincture (USP)
Iodine topical solution (USP)
Mercufenol chloride
Methylbenzethonium chloride
Nonylphenoxypoly (ethyleneoxy) ethanoliodine
Phenol
Poloxamer-iodine complex
Secondary amyltricresols
Sodium oxychlorosene
Tribromsalan
Triclocarban
Triclosan
Triple dye
Undecoylium chloride iodine complex
Combination of calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative
Combination of mercufenol chloride and secondary amyltricresols in 50 percent alcohol
* * * * *(d) * * *
(42) December 20, 2018, for products subject to paragraphs (a)(27)(vi) through (x) of this section.
End Signature End Supplemental InformationDated: December 14, 2017.
Leslie Kux,
Associate Commissioner for Policy.
Footnotes
1. Because the category of products referred to as “patient preoperative skin preparations” in the 1994 TFM and the 2015 Health Care Antiseptic PR encompasses products that are used for preinjection skin preparation in health care settings outside the hospital (so not preoperative), in this final rule we refer to such products as “patient antiseptic skin preparations.”
Back to Citation2. Also, note that drugs initially marketed in the United States after the OTC Drug Review began in 1972 and drugs without any U.S. marketing experience can be considered in the OTC monograph system based on submission of a time and extent application. (See § 330.14.)
Back to Citation3. General information about ASTM International can be found at https://www.astm.org/.
Back to Citation[FR Doc. 2017-27317 Filed 12-19-17; 8:45 am]
BILLING CODE 4164-01-P
Document Information
- Effective Date:
- 12/20/2018
- Published:
- 12/20/2017
- Department:
- Food and Drug Administration
- Entry Type:
- Rule
- Action:
- Final rule.
- Document Number:
- 2017-27317
- Dates:
- This rule is effective December 20, 2018.
- Pages:
- 60474-60503 (30 pages)
- Docket Numbers:
- Docket No. FDA-2015-N-0101
- RINs:
- 0910-AH40: Safety and Effectiveness of Healthcare Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use
- RIN Links:
- https://www.federalregister.gov/regulations/0910-AH40/safety-and-effectiveness-of-healthcare-antiseptics-topical-antimicrobial-drug-products-for-over-the-
- Topics:
- Administrative practice and procedure, Drugs, Labeling, Medical devices, Reporting and recordkeeping requirements
- PDF File:
- 2017-27317.pdf
- Supporting Documents:
- » 057 Reference 46 - Noguchi et al, Susceptibilities to antiseptic agents and distribution of antiseptic-resistance genes qacAB and smr of methicillin-resistant Staphylococcus aureus re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrob
- » 089 Reference 78 - FDA Regulatory Impact Analysis, Safety and Effectiveness for Health Care Antiseptics re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use
- » 088 Reference 77 - Joynson et al, Adaptive Resistance to Benzalkonium Chloride, Amikacin and Tobramycin re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use
- » 087 Reference 76 - Langsrud et al, Cross-Resistance to Antibiotics of Escherichia Coli Adapted to Benzalkonium Chloride or re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use
- » 086 Reference 75 - Braoudaki et al, Adaptive Resistance to Biocides in Salmonella Enterica and Escherichia Coli O157 and Cross-Resistance re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Huma
- » 085 Reference 74 - Copitch et al, Prevalence of decreased susceptibility to triclosan in Salmonella enterica isolates from animals and humans and association with multiple re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug
- » 084 Reference 73 - Morrissey et al, Evaluation of Epidemiological Cut-Off Values Indicates that Biocide Resistant Subpopulations Are Uncommon in Natural Isolates of Clinically re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial D
- » 083 Reference 72 - Lambert, Comparative Analysis of Antibiotic and Antimicrobial Biocide Susceptibility Data in Clinical Isolates of Methicillin-Sensitive Staphylococcus Aureus re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial
- » 082 Reference 71 - Shi et al, Prevalence of Antiseptic-Resistance Genes in Staphylococci Isolated from Orthokeratology Lens and Spectacle re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Huma
- » 081 Reference 70 - Buffet-Bataillon et al, Effect of higher minimum inhibitory concentrations of quaternary ammonium compounds in clinical E. coli isolates on antibiotic re Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Pr
- CFR: (1)
- 21 CFR 310.545