2024-16907. Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities; Updates to the Quality Reporting Program and Value-Based Purchasing Program for Federal Fiscal Year 2025
-
AGENCY:
Centers for Medicare & Medicaid Services (CMS), Department of Health and Human Services (HHS).
ACTION:
Final rule.
SUMMARY:
This final rule finalizes changes and updates to the policies and payment rates used under the Skilled Nursing Facility (SNF) Prospective Payment System (PPS) for fiscal year (FY) 2025. First, we are rebasing and revising the SNF market basket to reflect a 2022 base year. Next, we update the wage index used under the SNF PPS to reflect data collected during the most recent decennial census. Additionally, we finalize several technical revisions to the code mappings used to classify patients under the Patient Driven Payment Model (PDPM) to improve payment and coding accuracy. This final rule also updates the requirements for the SNF Quality Reporting Program and the SNF Value-Based Purchasing Program. Finally, we also are revising CMS' enforcement authority for imposing civil money penalties (CMPs) and including revisions to strengthen nursing home enforcement regulations.
DATES:
These regulations are effective on October 1, 2024.
FOR FURTHER INFORMATION CONTACT:
PDPM@cms.hhs.gov for issues related to the SNF PPS.
Heidi Magladry, (410) 786-6034, for information related to the skilled nursing facility quality reporting program.
Christopher Palmer, (410) 786-8025, for information related to the skilled nursing facility value-based purchasing program.
Celeste Saunders, (410) 786-5603, for information related to Nursing Home Enforcement.
SUPPLEMENTARY INFORMATION:
Availability of Certain Tables Exclusively Through the Internet on the CMS Website
As discussed in the FY 2014 SNF PPS final rule (78 FR 47936), tables setting forth the Wage Index for Urban Areas Based on Core-Based Statistical Area (CBSA) Labor Market Areas and the Wage Index Based on CBSA Labor Market Areas for Rural Areas are no longer published in the Federal Register . Instead, these tables are available exclusively through the internet on the CMS website. The wage index tables for this final rule can be accessed on the SNF PPS Wage Index home page, at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/WageIndex.html.
Readers who experience any problems accessing any of these online SNF PPS wage index tables should contact Kia Burwell at (410) 786-7816.
To assist readers in referencing sections contained in this document, we are providing the following Table of Contents.
Table of Contents
I. Executive Summary
A. Purpose
B. Summary of Major Provisions
C. Summary of Cost and Benefits
II. Background on SNF PPS
A. Statutory Basis and Scope
B. Initial Transition for the SNF PPS
C. Required Annual Rate Updates
III. Analysis and Responses to Public Comments on the FY 2025 SNF PPS Proposed Rule
A. General Comments on the FY 2025 SNF PPS Proposed Rule
IV. SNF PPS Rate Setting Methodology and FY 2025 Update
A. Federal Base Rates
B. SNF Market Basket Update
C. Case-Mix Adjustment
D. Wage Index Adjustment
E. SNF Value-Based Purchasing Program
F. Adjusted Rate Computation Example
V. Additional Aspects of the SNF PPS
A. SNF Level of Care—Administrative Presumption
B. Consolidated Billing
C. Payment for SNF-Level Swing-Bed Services
VI. Other SNF PPS Issues
A. Rebasing and Revising the SNF Market Basket
B. Changes to SNF PPS Wage Index
C. Technical Updates to PDPM ICD-10 Mappings
D. Request for Information: Update to PDPM Non-Therapy Ancillary Component
VII. Skilled Nursing Facility Quality Reporting Program (SNF QRP)
A. Background and Statutory Authority
B. General Considerations Used for the Selection of Measures for the SNF QRP
C. Collection of Four Additional Items as Standardized Patient Assessment Data Elements and Modification of One Item Collected as a Standardized Patient Assessment Data Element Beginning With the FY 2027 SNF QRP
D. SNF QRP Quality Measure Concepts Under Consideration for Future Years—Request for Information (RFI)
E. Form, Manner, and Timing of Data Submission Under the SNF QRP
F. Policies Regarding Public Display of Measure Data for the SNF QRP
VIII. Skilled Nursing Facility Value-Based Purchasing (SNF VBP) Program
A. Statutory Background
B. Regulation Text Technical Updates
C. SNF VBP Program Measures
D. SNF VBP Performance Standards
E. SNF VBP Performance Scoring Methodology
F. Updates to the SNF VBP Review and Correction Process
G. Updates to the SNF VBP Extraordinary Circumstances Exception Policy
IX. Nursing Home Enforcement
A. Background
B. Analysis of the Provisions of the Proposed Regulations
X. Collection of Information Requirements
XI. Economic Analyses
A. Regulatory Impact Analysis
B. Regulatory Flexibility Act Analysis
C. Unfunded Mandates Reform Act Analysis
D. Federalism Analysis
E. Regulatory Review Costs
I. Executive Summary
A. Purpose
This final rule will update the SNF prospective payment rates for fiscal year (FY) 2025, as required under section 1888(e)(4)(E) of the Social Security Act (the Act). It also responds to section 1888(e)(4)(H) of the Act, which requires the Secretary to provide for publication of certain specified information relating to the payment update (see section II.C. of this final rule) in the Federal Register before the August 1 that precedes the start of each FY. Additionally, in this final rule, we are finalizing the rebasing and revising of the SNF market basket to reflect a 2022 base year. Next, we are finalizing the update to the wage index used under the SNF PPS to reflect data collected during the most recent decennial census. We also finalize several technical revisions to the code mappings used to classify patients under the PDPM to improve payment and coding accuracy. This final rule updates the requirements for the SNF QRP, including the collection of four new items as standardized patient assessment data elements, and the modification of one item collected and submitted using the Minimum Data Set (MDS) beginning with the FY 2027 SNF QRP. We also finalize a policy that SNFs, which participate in the SNF QRP, participate in a validation process beginning with the FY 2027 SNF QRP. We also provide a summary of the comments received on the request for information on quality measure concepts under consideration for future SNF QRP program years. This final rule also includes requirements for the Skilled Nursing Facility Value-Based Purchasing (SNF VBP) Program, including adopting a measure selection, retention, and removal policy, a technical measure updates policy, a measure minimum for FY 2028 and subsequent years, updates to the review and correction policy to accommodate new measure data sources, updates to the Extraordinary Circumstances Exception policy, and updates to the SNF VBP regulation text. We also proposed revisions to existing long-term care (LTC) enforcement regulations that would enable CMS and the States to impose CMPs to better reflect amounts that are more consistent with the type of noncompliance that occurred.
B. Summary of Major Provisions
In accordance with sections 1888(e)(4)(E)(ii)(IV) and (e)(5) of the Act, this final rule updates the annual rates that we published in the SNF PPS final rule for FY 2024 (88 FR 53200, August 7, 2023). In addition, this final rule includes a forecast error adjustment for FY 2025. We are also finalizing the rebasing and revising of the SNF market basket to reflect a 2022 base year. Next, we are finalizing the update of the wage index used under the SNF PPS to reflect data collected during the most recent decennial census. We are also finalizing several technical revisions to the code mappings used to classify patients under the PDPM to improve payment and coding accuracy.
We are finalizing several updates for the SNF VBP Program. We are adopting a measure selection, retention, and removal policy that aligns with policies we have adopted in other CMS quality programs. We are adopting a technical measure updates policy that allows us to incorporate technical measure updates into SNF VBP measure specifications and to update the numerical values of the performance standards for a program year if a measure's specifications were technically updated between the time that we published the performance standards for a measure and the time that we calculate SNF performance on that measure at the conclusion of the applicable performance period. We are adopting the same measure minimum we previously finalized for the FY 2027 program year for the FY 2028 program year and subsequent program years. We are adopting modifications to Phase One of our review and correction policy such that the policy applies to all SNF VBP measures regardless of the measure's data source. We are updating the SNF VBP extraordinary circumstances exception (ECE) policy to allow SNFs to request an ECE if the SNF can demonstrate that, as a result of the extraordinary circumstance, it cannot report SNF VBP data on one or more measures by the specified deadline. We are also updating the instructions for requesting an extraordinary circumstance exception (ECE). Lastly, we are adopting several updates to the SNF VBP regulation text to align with previously finalized definitions and policies.
Beginning with the FY 2027 SNF QRP, we are finalizing requirements that SNFs participating in the SNF QRP collect and submit through the MDS four new items as standardized patient assessment data elements under the social determinants of health (SDOH) category: one item for Living Situation, two items for Food, and one item for Utilities. Additionally, we are finalizing our proposal to modify the current Transportation item. We are finalizing with modification a validation process for the SNF QRP, similar to the process that we adopted for the SNF VBP beginning with the FY 2027 SNF QRP. We are also finalizing with modification amendments to the regulation text at § 413.360 to implement the validation process we are finalizing. Finally, this final rule also summarizes comments we received in response to a request for information (RFI) on quality measure concepts under consideration for future SNF QRP years.
We are finalizing revisions to CMS' existing enforcement authority to expand the number and types of CMPs that can be imposed on LTC facilities, allowing for more per-instance (PI) CMPs to be imposed in conjunction with per-day (PD) CMPs. This update also expands our authority to impose multiple PI CMPs when the same type of noncompliance is identified on more than one day. Lastly, the final revisions will enable CMS or the States to impose a CMP for the number of days of previously cited noncompliance since the last three standard surveys for which a CMP has not yet been imposed to ensure that identified noncompliance may be subject to a penalty.
C. Summary of Cost and Benefits
II. Background on SNF PPS
A. Statutory Basis and Scope
As amended by section 4432 of the Balanced Budget Act of 1997 (BBA 1997) (Pub. L. 105-33, enacted August 5, 1997), section 1888(e) of the Act provides for the implementation of a PPS for SNFs. This methodology uses prospective, case-mix adjusted per diem payment rates applicable to all covered SNF services defined in section 1888(e)(2)(A) of the Act. The SNF PPS is effective for cost reporting periods beginning on or after July 1, 1998, and covers virtually all costs of furnishing covered SNF services (routine, ancillary, and capital-related costs) other than costs associated with approved educational activities and bad debts. Under section 1888(e)(2)(A)(i) of the Act, covered SNF services include post-hospital extended care services for which benefits are provided under Part A, as well as those items and services (other than a small number of excluded services, such as physicians' services) for which payment may otherwise be made under Part B and which are furnished to Medicare beneficiaries who are residents in a SNF during a covered Part A stay. A comprehensive discussion of these provisions appears in the May 12, 1998, interim final rule (63 FR 26252). In addition, a detailed discussion of the legislative history of the SNF PPS is available online at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/Downloads/Legislative_History_2018-10-01.pdf.
Section 215(a) of the Protecting Access to Medicare Act of 2014 (PAMA) (Pub. L. 113-93, enacted April 1, 2014) added section 1888(g) to the Act, requiring the Secretary to specify an all-cause all-condition hospital readmission measure and an all-condition risk-adjusted potentially preventable hospital readmission measure for the SNF setting. Additionally, section 215(b) of PAMA added section 1888(h) to the Act requiring the Secretary to implement a VBP program for SNFs. In 2014, section 2(c)(4) of the Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014 (Pub. L. 113-185, enacted October 6, 2014) amended section 1888(e)(6) of the Act, which requires the Secretary to implement a QRP for SNFs under which SNFs report data on measures and resident assessment data. Finally, section 111 of the Consolidated Appropriations Act, 2021 (CAA, 2021) (Pub. L. 116-260, enacted December 27, 2020) amended section 1888(h) of the Act, authorizing the Secretary to apply up to nine additional measures to the VBP program for SNFs.
B. Initial Transition for the SNF PPS
Under sections 1888(e)(1)(A) and (e)(11) of the Act, the SNF PPS included an initial, three-phase transition that blended a facility-specific rate (reflecting the individual facility's historical cost experience) with the Federal case-mix adjusted rate. The transition extended through the facility's first 3 cost reporting periods under the PPS, up to and including the one that began in FY 2001. Thus, the SNF PPS is no longer operating under the transition, as all facilities have been paid at the full Federal rate effective with cost reporting periods beginning in FY 2002. As we now base payments for SNFs entirely on the adjusted Federal per diem rates, we no longer include adjustment factors under the transition related to facility-specific rates for the upcoming FY.
C. Required Annual Rate Updates
Section 1888(e)(4)(E) of the Act requires the SNF PPS payment rates to be updated annually. The most recent annual update occurred in a final rule that set forth updates to the SNF PPS payment rates for FY 2024 (88 FR 53200, August 7, 2023), as amended by the subsequent correction document (88 FR 68486, October 4, 2023).
Section 1888(e)(4)(H) of the Act specifies that we provide for publication annually in the Federal Register the following:
- The unadjusted Federal per diem rates to be applied to days of covered SNF services furnished during the upcoming FY.
- The case-mix classification system to be applied for these services during the upcoming FY.
- The factors to be applied in making the area wage adjustment for these services.
Along with other revisions discussed later in this preamble, this final rule will set out the required annual updates to the per diem payment rates for SNFs for FY 2025.
III. Analysis and Responses to Public Comments on the FY 2025 SNF PPS Proposed Rule
A. General Comments on the FY 2025 SNF PPS Proposed Rule
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: Some commenters expressed concerns regarding several items outside the scope of this rule or outside the scope of CMS's current authorities. These comments included issues related to the recently finalized nursing home staffing rule (outside of issues related to that rule and calculation of the SNF market basket, which are addressed later in this rule), and a request that CMS remove the 3-day qualifying hospital stay (QHS) prerequisite for Part A SNF coverage.
Response: With regard to those comments related to the recently finalized nursing home staffing rule, any such issues are out of scope for this rule and should be directed to HealthandSafetyInquiries@cms.hhs.gov. With regard to the request that we remove the QHS requirement for Part A SNF coverage, we maintain that we do not have the statutory authority to pursue this change at this time. Moreover, we have previously conducted analyses of the associated cost of removing the 3-day stay requirement and found that it would significantly increase Medicare outlays.
Comment: Several commenters raised concerns with therapy treatment under PDPM, specifically related to reductions in the amount of therapy furnished to SNF patients since PDPM was implemented. Some of these commenters stated that CMS should revise the existing limit on concurrent and group therapy to provide a financial penalty in cases where the facility exceeds this limit. These commenters also recommended that CMS direct its review contractors to examine the practices of facilities that changed their therapy service provision after PDPM was implemented. Additionally, commenters want CMS to release the results of any monitoring efforts around therapy provision. Some commenters stated that the therapy items in O0400 should be maintained to track therapy provision. Finally, some commenters stated that CMS should reinstate the assessment schedule that had existed prior to implementing PDPM.
Response: We appreciate commenters raising these concerns around therapy provision under PDPM, as compared the Resource Utilization Groups, Version IV (RUG-IV). We agree with commenters that the amount of therapy that is furnished to patients under PDPM is less than that delivered under RUG-IV. As we stated in the FY 2020 SNF PPS final rule, we believe that close, real-time monitoring is essential to identifying any adverse trends under PDPM. While we have identified the same reduction in therapy services and therapy staff, we believe that these findings must be considered within the context of patient outcomes. To the extent that facilities are able to maintain or improve patient outcomes, we believe that this supersedes changes in service provision, whether this be in the amount of therapy furnished or the mode in which it is furnished. We continue to monitor all aspects of PDPM and advise our review contractors on any adverse trends. With regard to implementing a specific penalty for exceeding the group and concurrent therapy threshold, based on our current data, we have not identified any widespread misuse of this limit. Should we identify such misuse, either at a provider-level or at a broader level, we will pursue an appropriate course of action.
With regard to eliminating certain therapy tracking items in O0400, while the O0400 items are able to track therapy minutes, these items only track therapy provision for the seven days up to and including the assessment reference date. We agree with the commenters that items should exist to track therapy provision over the course of a full Medicare stay, which is the purpose of the O0425 items on the assessment.
Finally, with regard to the recommendation that we reinstate something akin to the assessment schedule that was in effect under RUG- IV, given that PDPM does not reimburse on the basis of therapy minutes, we do not believe that such an increase in administrative burden on providers would have an impact on therapy provision. That being said, we strongly encourage interested parties to continue to provide suggestions on how to ensure that SNF patients receive the care they need based on their unique characteristics and goals.
Comment: One commenter requested that we consider including recreational therapy time provided to SNF residents by recreational therapists into the case- mix adjusted therapy component of PDPM, rather than having it be considered part of the nursing component. This commenter further suggested that CMS begin collecting data, as part of a demonstration project, on the utilization of recreational therapy, as a distinct and separate service, and its impact on patient care cost and quality.
Response: We appreciate the commenter raising this issue, but we do not believe there is sufficient evidence at this time regarding the efficacy of recreational therapy interventions. More notably, we do not believe there are data that would substantiate a determination of the effect on payment of such interventions, as such services were not considered separately when the PDPM was being developed, unlike physical, occupational and speech-language pathology services. That being said, we would note that Medicare Part A originally paid for institutional care in various provider settings, including SNF, on a reasonable cost basis, but now makes payment using PPS methodologies, such as the SNF PPS. To the extent that one of these SNFs furnished recreational therapy to its inpatients under the previous, reasonable cost methodology, the cost of the services would have been included in the base payments when SNF PPS payment rates were derived. Under the PPS methodology, Part A makes a comprehensive payment for the bundled package of items and services that the facility furnishes during the course of a Medicare-covered stay. This package encompasses nearly all services that the beneficiary receives during the course of the stay—including any medically necessary recreational therapy—and payment for such services is included within the facility's comprehensive SNF PPS payment for the covered Part A stay itself. With regard to developing a demonstration project focused on this particular service, we do not believe that creating such a project would substantially improve the accuracy of the SNF PPS payment rates. Moreover, in light of comments discussed previously in this section on the impact of PDPM implementation on therapy provision more generally, we believe that carving out recreational therapy as a separate discipline will not have a significant impact on access to recreational therapy services for SNF patients.
IV. SNF PPS Rate Setting Methodology and FY 2025 Payment Update
A. Federal Base Rates
Under section 1888(e)(4) of the Act, the SNF PPS uses per diem Federal payment rates based on mean SNF costs in a base year (FY 1995) updated for inflation to the first effective period of the PPS. We developed the Federal payment rates using allowable costs from hospital-based and freestanding SNF cost reports for reporting periods beginning in FY 1995. The data used in developing the Federal rates also incorporated a Part B add-on, which is an estimate of the amounts that, prior to the SNF PPS, would be payable under Part B for covered SNF services furnished to individuals during the course of a covered Part A stay in a SNF.
In developing the rates for the initial period, we updated costs to the first effective year of the PPS (the 15-month period beginning July 1, 1998) using the SNF market basket, and then standardized for geographic variations in wages and for the costs of facility differences in case-mix. In compiling the database used to compute the Federal payment rates, we excluded those providers that received new provider exemptions from the routine cost limits, as well as costs related to payments for exceptions to the routine cost limits. Using the formula that the BBA 1997 prescribed, we set the Federal rates at a level equal to the weighted mean of freestanding costs plus 50 percent of the difference between the freestanding mean and weighted mean of all SNF costs (hospital-based and freestanding) combined. We computed and applied separately the payment rates for facilities located in urban and rural areas and adjusted the portion of the Federal rate attributable to wage-related costs by a wage index to reflect geographic variations in wages.
B. SNF Market Basket Update
1. SNF Market Basket
Section 1888(e)(5)(A) of the Act requires us to establish a SNF market basket that reflects changes over time in the prices of an appropriate mix of goods and services included in covered SNF services. Accordingly, we have developed a SNF market basket that encompasses the most commonly used cost categories for SNF routine services, ancillary services, and capital-related expenses. In the SNF PPS final rule for FY 2022 (86 FR 42444 through 42463), we rebased and revised the SNF market basket, which included updating the base year from 2014 to 2018. In the SNF PPS proposed rule for FY 2025 (89 FR 23427 through 23451), we proposed to rebase and revise the SNF market basket and update the base year from 2018 to 2022. We are finalizing the 2022-based SNF market basket as proposed, as discussed in section VI.A. of this final rule. The SNF market basket is used to compute the market basket percentage increase that is used to update the SNF Federal rates on an annual basis, as required by section 1888(e)(4)(E)(ii)(IV) of the Act. This market basket percentage increase is adjusted by a forecast error adjustment, if applicable, and then further adjusted by the application of a productivity adjustment as required by section 1888(e)(5)(B)(ii) of the Act and described in section IV.B.4. of this final rule.
As outlined in the proposed rule, we proposed a FY 2025 SNF market basket percentage increase of 2.8 percent based on IHS Global Inc.'s (IGI's) fourth-quarter 2023 forecast of the proposed 2022-based SNF market basket (before application of the forecast error adjustment and productivity adjustment). We also proposed that if more recent data subsequently became available (for example, a more recent estimate of the market basket and/or the productivity adjustment), we would use such data, if appropriate, to determine the FY 2025 SNF market basket percentage increase, labor-related share relative importance, forecast error adjustment, or productivity adjustment in this SNF PPS final rule.
Since the proposed rule, we have updated the FY 2025 market basket percentage increase based on IGI's second quarter 2024 forecast with historical data through the first quarter of 2024. The FY 2025 growth rate of the 2022-based SNF market basket is estimated to be 3.0 percent.
2. Market Basket Update for FY 2025
Section 1888(e)(5)(B) of the Act defines the SNF market basket percentage increase as the percentage change in the SNF market basket from the midpoint of the previous FY to the midpoint of the current FY. For the Federal rates outlined in the proposed rule, we used the percentage change in the SNF market basket to compute the update factor for FY 2025. This factor was based on the FY 2025 percentage increase in the proposed 2022-based SNF market basket reflecting routine, ancillary, and capital-related expenses. Sections 1888(e)(4)(E)(ii)(IV) and (e)(5)(B)(i) of the Act require that the update factor used to establish the FY 2025 unadjusted Federal rates be at a level equal to the SNF market basket percentage increase. Accordingly, we determined the total growth from the average market basket level for the period of October 1, 2023, through September 30, 2024, to the average market basket level for the period of October 1, 2024, through September 30, 2025. As outlined in the proposed rule, we proposed a FY 2025 SNF market basket percentage increase of 2.8 percent. For this final rule, based on IGI's second quarter 2024 forecast with historical data through the first quarter of 2024, the FY 2025 growth rate of the 2022-based SNF market basket is estimated to be 3.0 percent.
As further explained in section IV.B.3. of this final rule, as applicable, we adjust the percentage increase by the forecast error adjustment from the most recently available FY for which there is final data and apply this adjustment whenever the difference between the forecasted and actual percentage increase in the market basket exceeds a 0.5 percentage point threshold in absolute terms. Additionally, section 1888(e)(5)(B)(ii) of the Act requires us to reduce the market basket percentage increase by the productivity adjustment (the 10-year moving average of changes in annual economy-wide private nonfarm business total factor productivity (TFP) for the period ending September 30, 2025) which is estimated to be 0.5 percentage point, as described in section IV.B.4. of this final rule.
We also note that section 1888(e)(6)(A)(i) of the Act provides that, beginning with FY 2018, SNFs that fail to submit data, as applicable, in accordance with sections 1888(e)(6)(B)(i)(II) and (III) of the Act for a fiscal year will receive a 2.0 percentage point reduction to their market basket update for the fiscal year involved, after application of section 1888(e)(5)(B)(ii) of the Act (the productivity adjustment) and section 1888(e)(5)(B)(iii) of the Act (the market basket increase). In addition, section 1888(e)(6)(A)(ii) of the Act states that application of the 2.0 percentage point reduction (after application of section 1888(e)(5)(B)(ii) and (iii) of the Act) may result in the market basket percentage change being less than zero for a fiscal year and may result in payment rates for a fiscal year being less than such payment rates for the preceding fiscal year. Section 1888(e)(6)(A)(iii) of the Act further specifies that the 2.0 percentage point reduction is applied in a noncumulative manner, so that any reduction made under section 1888(e)(6)(A)(i) of the Act applies only to the fiscal year involved, and that the reduction cannot be taken into account in computing the payment amount for a subsequent fiscal year.
The following is a of the public comments received on the proposed FY 2025 SNF market basket percentage increase to the SNF PPS rates, along with our responses.
Comment: Many commenters stated that they appreciate and support the proposed net 4.1 percent payment update and forecast error adjustment; however, some commenters expressed concerns about missed forecasts and whether the market basket is appropriately capturing inflation.
Commenters cited a report from the AHA, which found that hospital employee compensation has grown by 45 percent since 2014, and workforce shortages that may persist into the future could continue to drive labor-related inflation higher. As a result, providers have turned to more expensive contract labor to sustain operations. Several commenters noted themselves or their members experiencing high rates of inflation in equipment and supplies, and questioned whether the inflation is being properly captured in the market basket.
A few commenters noted that there have now been four consecutive years of under-forecasts, and that growth in the Consumer Price Index All Urban totaled 16.8 percent between 2021 and 2023 while SNF market basket growth totaled only 15.5 percent over the same time period. Several commenters also expressed that the proposed 4.1 percent payment update will fall short of covering the costs of the finalized minimum staffing rule. Two commenters urged CMS to consider a prospective adjustment for labor inflation. Two commenters urged CMS to use more recent data to determine the FY SNF market basket update in the final rule.
Response: We recognize commenters' concerns in relation to forecast error during a high inflationary period. SNF PPS market basket updates are set prospectively, which means that the market basket update relies on a mix of both historical data for part of the period for which the update is calculated and forecasted data for the remainder. For instance, the FY 2025 market basket update in this final rule reflects historical data through the first quarter of 2024 and forecasted data through the third quarter of 2025. IHS Global Inc. (IGI) is a nationally recognized economic and financial forecasting firm with which CMS contracts to forecast the components of the market baskets. We believe that basing the prospective update on these forecasts is an appropriate method, while also acknowledging that these are expectations of trends and may differ from actual experience.
We also understand commenters' concerns regarding the minimum staffing rule not being taken into account. The 2022-based SNF market basket is a fixed-weight, Laspeyres-type price index that measures the change in price, over time, of the same mix of goods and services purchased in the base period. Any changes in the quantity or mix of goods and services (that is, intensity) purchased over time relative to a base period are not measured. The cost weights in this final rule are based on the most recent set of complete and comprehensive cost data for the universe of SNF providers available at the time of rulemaking, and the price proxies for each cost category include expectations of the inflationary pressures for each category of expenses in the market basket. Any changes in intensity relative to the 2022-based SNF market basket will be reflected in future Medicare cost reports and thus captured in the next rebasing. We will continue to monitor Medicare cost report data for freestanding SNFs as it becomes available to assess whether the 2022-based SNF market basket cost weights continue to be appropriate in the coming years.
We recognize the challenges facing SNFs in operating during a high inflationary environment. Due to SNF payments under PPS being set prospectively, we rely on a projection of the SNF market basket that reflects both recent historical trends, as well as forecast expectations over the next 18 months. The forecast error for a market basket update is calculated as the actual market basket increase for a given year, less the forecasted market basket increase. Due to the uncertainty regarding future price trends, forecast errors can be both positive or negative. We are confident that the forecast error adjustments built into the SNF market basket update factor will account for these discrepancies over time.
The proposed FY 2025 SNF market basket percentage increase of 2.8 percent reflected the most-recent forecast available at that time of rulemaking. As stated in the SNF PPS proposed rule for FY 2025 (89 FR 23451), we also proposed that if more recent data subsequently became available (for example, a more recent estimate of the market basket and/or the productivity adjustment), we would use such data, if appropriate, to determine the FY 2025 SNF market basket percentage increase, labor-related share relative importance, forecast error adjustment, or productivity adjustment in the SNF PPS final rule. For this final rule, we have incorporated the most recent historical data and forecasts provided by IGI to capture the expected price and wage pressures facing SNFs in FY 2025. For this final rule, based on IGI's second-quarter 2024 forecast with historical data through first-quarter 2024, the FY 2025 growth rate of the 2022-based SNF market basket is 3.0 percent. By incorporating the most recent estimates available of the market basket percentage increase, we believe these data reflect the best available projection of input price inflation faced by SNFs in FY 2025.
After consideration of the comments received on the FY 2025 SNF market basket proposals, we are finalizing a FY 2025 SNF market basket percentage increase of 3.0 percent (prior to the application of the forecast error adjustment and productivity adjustment, which are discussed later in this section).
3. Forecast Error Adjustment
As discussed in the June 10, 2003 supplemental proposed rule (68 FR 34768) and finalized in the August 4, 2003 final rule (68 FR 46057 through 46059), § 413.337(d)(2) provides for an adjustment to account for market basket forecast error. The initial adjustment for market basket forecast error applied to the update of the FY 2003 rate for FY 2004 and took into account the cumulative forecast error for the period from FY 2000 through FY 2002, resulting in an increase of 3.26 percent to the FY 2004 update. Subsequent adjustments in succeeding FYs take into account the forecast error from the most recently available FY for which there is final data and apply the difference between the forecasted and actual change in the market basket when the difference exceeds a specified threshold. We originally used a 0.25 percentage point threshold for this purpose; however, for the reasons specified in the FY 2008 SNF PPS final rule (72 FR 43425), we adopted a 0.5 percentage point threshold effective for FY 2008 and subsequent FYs. As we stated in the final rule for FY 2004 that first issued the market basket forecast error adjustment (68 FR 46058), the adjustment will reflect both upward and downward adjustments, as appropriate.
For FY 2023 (the most recently available FY for which there is final data), the forecasted or estimated increase in the SNF market basket was 3.9 percent, and the actual increase for FY 2023 was 5.6 percent, resulting in the actual increase being 1.7 percentage points higher than the estimated increase. Accordingly, as the difference between the estimated and actual amount of change in the market basket exceeds the 0.5 percentage point threshold, under the policy previously described (comparing the forecasted and actual market basket percentage increase), the FY 2025 market basket percentage increase of 3.0 percent is adjusted upward to account for the forecast error adjustment of 1.7 percentage points, resulting in a SNF market basket percentage increase of 4.7 percent, which is then reduced by the productivity adjustment of 0.5 percentage point, discussed in section IV.B.4. of this final rule. This results in a SNF market basket update for FY 2025 of 4.2 percent.
Table 2 shows the forecasted and actual market basket increases for FY 2023.
A discussion of the public comments received on the forecast error adjustment, along with our responses, can be found below.
Comment: Several commenters noted that while they appreciate the forecast error adjustment, forecast error adjustments are made two years after the year in question and SNFs must contend with the underpayment for two years before it is reconciled. One commenter suggested updating the method to use more timely data that would capture increased costs in recent years.
Response: While we understand that earlier forecast error adjustments might be preferable, a two-year lag is necessary because historical data for the current fiscal year are not available until after the following year's update is determined.
Comment: One commenter stated that not including Federal relief funds, the aggregate fee-for-service (FFS) Medicare margin for freestanding SNFs in 2022 was over 18 percent, the 23rd consecutive year this this margin has exceeded 10 percent. They note that high margins indicate that a reduction is needed to more closely align aggregate payments to aggregate costs.
The commenter also noted that although CMS is required by statute to update the payment rates each year by the estimated change in the market basket, CMS is not required to make automatic forecast error corrections. They maintain that they do not support forecast error adjustments for three reasons. First, in some years, such as the one addressed by the proposed rule for FY 2025, the forecast error correction results in making a larger payment increase in addition to the statutory update, even as the aggregate FFS Medicare margin is high. Second, the adjustments result in more variable updates than had no adjustment been made. Since FY 2004, when CMS implemented the adjustment, forecast error corrections have ranged from a 3.26 percent increase (in FY 2004) to a −0.8 percent reduction (in FY 2022). Eliminating the adjustment for forecast errors would result in more stable updates. Third, the adjustment results in inconsistent approaches to updates across settings: except for the updates to the capital payments to acute care hospitals, CMS does not apply forecast error adjustments to any other market basket updates.
Response: We appreciate the commenter's input and suggestions. We note that apart from the last several years of various unprecedented market shocks and resulting volatility, forecast errors have generally been relatively small and clustered near zero. We agree that forecast error adjustments have potential to introduce more variable and unstable updates. As a result, for FY 2008 and subsequent years we increased the threshold at which adjustments are triggered from 0.25 percentage point to 0.5 percentage point. Our intent in raising the threshold was to distinguish typical statistical variances from more major unanticipated impacts, such as unforeseen disruptions of the economy or unexpected inflationary patterns.
As was stated when the SNF forecast error adjustment was introduced in the FY 2004 SNF PPS final rule (68 FR 46035), our goal continues to be to “pay the appropriate amount, to the correct provider, for the proper service, at the right time.” Accordingly, we are optimistic that market volatility will soon subside to a point where forecast errors will not be frequently triggered. Nonetheless, we will continue to monitor the effects of forecast error adjustments, and their appropriateness in responding to unforeseen inflationary patterns. Any changes, if deemed necessary, would be proposed through notice and comment rulemaking.
After consideration of the comments received, we are finalizing the application of the proposed forecast error adjustment without modification. As stated above, based on IGI's second-quarter 2024 forecast with historical data through the first quarter of 2024, the FY 2025 growth rate of the 2022-based SNF market basket is estimated to be 3.0 percent. Accordingly, as the difference between the estimated and actual amount of change in the market basket exceeds the 0.5 percentage point threshold, under the policy previously described (comparing the forecasted and actual market basket percentage increase), the FY 2025 market basket percentage increase of 3.0 percent is adjusted upward to account for the forecast error adjustment of 1.7 percentage points, resulting in a SNF market basket percentage increase of 4.7 percent, which is then reduced by the productivity adjustment as discussed later in this section.
4. Productivity Adjustment
Section 1888(e)(5)(B)(ii) of the Act, as added by section 3401(b) of the Patient Protection and Affordable Care Act (Affordable Care Act) (Pub. L. 111-148, enacted March 23, 2010) requires that, in FY 2012 and in subsequent FYs, the market basket percentage under the SNF payment system (as described in section 1888(e)(5)(B)(i) of the Act) is to be reduced annually by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act. Section 1886(b)(3)(B)(xi)(II) of the Act, in turn, defines the productivity adjustment to be equal to the 10-year moving average of changes in annual economy-wide, private nonfarm business multifactor productivity (MFP) (as projected by the Secretary for the 10-year period ending with the applicable FY, year, cost-reporting period, or other annual period).
The U.S. Department of Labor's Bureau of Labor Statistics (BLS) publishes the official measure of productivity for the U.S. We note that previously the productivity measure referenced at section 1886(b)(3)(B)(xi)(II) of the Act was published by BLS as private nonfarm business multifactor productivity. Beginning with the November 18, 2021, release of productivity data, BLS replaced the term MFP with TFP. BLS noted that this is a change in terminology only and will not affect the data or methodology. As a result of the BLS name change, the productivity measure referenced in section 1886(b)(3)(B)(xi)(II) of the Act is now published by BLS as private nonfarm business total factor productivity. We refer readers to the BLS website at www.bls.gov for the BLS historical published TFP data. A complete description of the TFP projection methodology is available on our website at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/MarketBasketResearch. In addition, in the FY 2022 SNF final rule (86 FR 42429) we noted that, effective with FY 2022 and forward, we changed the name of this adjustment to refer to it as the “productivity adjustment,” rather than the “MFP adjustment.”
Per section 1888(e)(5)(A) of the Act, the Secretary shall establish a SNF market basket that reflects changes over time in the prices of an appropriate mix of goods and services included in covered SNF services. Section 1888(e)(5)(B)(ii) of the Act, added by section 3401(b) of the Affordable Care Act, requires that for FY 2012 and each subsequent FY, after determining the market basket percentage described in section 1888(e)(5)(B)(i) of the Act, the Secretary shall reduce such percentage by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act. Section 1888(e)(5)(B)(ii) of the Act further states that the reduction of the market basket percentage by the productivity adjustment may result in the market basket percentage being less than zero for a FY and may result in payment rates under section 1888(e) of the Act being less than such payment rates for the preceding fiscal year. Thus, if the application of the productivity adjustment to the market basket percentage calculated under section 1888(e)(5)(B)(i) of the Act results in a productivity-adjusted market basket percentage that is less than zero, then the annual update to the unadjusted Federal per diem rates under section 1888(e)(4)(E)(ii) of the Act would be negative, and such rates would decrease relative to the prior FY.
Based on the data available for this FY 2025 SNF PPS final rule, the productivity adjustment (the 10-year moving average of changes in annual economy-wide private nonfarm business TFP for the period ending September 30, 2025) is projected to be 0.5 percentage point.
Comment: A few commenters noted that they are disappointed in the productivity adjustment, and that CMS should closely monitor the effect of such productivity adjustments and explore ways to use its authority to offset or waive them.
Response: Section 1888(e)(5)(B)(ii) of the Act requires the application of the productivity adjustment described in section 1886(b)(3)(xi)(II) of the Act to the SNF PPS market basket increase factor. As required by statute, the FY 2025 productivity adjustment is derived based on the 10-year moving average growth in economy-wide productivity for the period ending in FY 2025. We recognize the concerns of the commenters regarding the appropriateness of the productivity adjustment; however, we are required under section 1888(e)(5)(B)(ii) of the Act to apply the specific productivity adjustment described here in this section.
As stated previously, in the proposed rule the productivity adjustment was estimated to be 0.4 percentage point based on IGI's fourth-quarter 2024 forecast. For this final rule, based on IGI's second-quarter 2024 forecast, the productivity adjustment (the 10-year moving average of changes in annual economy-wide private nonfarm business TFP for the period ending September 30, 2025) is 0.5 percentage point.
Consistent with section 1888(e)(5)(B)(i) of the Act and § 413.337(d)(2), and as outlined previously in section IV.B.1. of this final rule, the market basket percentage increase for FY 2025 for the SNF PPS is based on IGI's second quarter 2024 forecast of the SNF market basket percentage increase, which is estimated to be 3.0 percent. This market basket percentage increase is then increased by 1.7 percentage points, due to application of the forecast error adjustment outlined earlier in section IV.B.3. of this final rule. Finally, as outlined earlier in this section, we are applying a 0.5 percentage point productivity adjustment to the FY 2025 SNF market basket percentage increase. Therefore, the resulting productivity-adjusted FY 2025 SNF market basket update is equal to 4.2 percent, which reflects a market basket percentage increase of 3.0 percent, plus the 1.7 percentage points forecast error adjustment, and reduced by the 0.5 percentage point productivity adjustment. Thus, we apply a net SNF market basket update factor of 4.2 percent in our determination of the FY 2025 SNF PPS unadjusted Federal per diem rates.
5. Unadjusted Federal Per Diem Rates for FY 2025
As discussed in the FY 2019 SNF PPS final rule (83 FR 39162), in FY 2020 we implemented a new case-mix classification system to classify SNF patients under the SNF PPS, the PDPM. As discussed in section V.B.1. of that final rule (83 FR 39189), under PDPM, the unadjusted Federal per diem rates are divided into six components, five of which are case-mix adjusted components (Physical Therapy (PT), Occupational Therapy (OT), Speech-Language Pathology (SLP), Nursing, and Non-Therapy Ancillaries (NTA)), and one of which is a non-case-mix component, as existed under the previous RUG-IV model. We proposed to use the SNF market basket, adjusted as outlined previously in sections III.B.1. through III.B.4. of the proposed rule, to adjust each per diem component of the Federal rates forward to reflect the change in the average prices for FY 2024 from the average prices for FY 2023. We also proposed to further adjust the rates by a wage index budget neutrality factor, outlined in section III.D. of the proposed rule.
Further, in the past, we used the revised Office of Management and Budget (OMB) delineations adopted in the FY 2015 SNF PPS final rule (79 FR 45632, 45634), with updates as reflected in OMB Bulletin Nos. 15-01 and 17-01, to identify a facility's urban or rural status for the purpose of determining which set of rate tables apply to the facility. As discussed in the FY 2021 SNF PPS proposed and final rules, we adopted the revised OMB delineations identified in OMB Bulletin No. 18-04 (available at https://www.whitehouse.gov/wp-content/uploads/2018/09/Bulletin-18-04.pdf) to identify a facility's urban or rural status effective beginning with FY 2021. However, as further outlined in section V.A of the proposed rule, the current CBSAs are based on OMB standards contained in Bulletin 20-01, which is based on data collected during the 2010 Decennial Census. In this final rule, we are updating the SNF PPS wage index using the CBSAs defined within Bulletin 23-01.
Tables 3 and 4 reflect the proposed unadjusted Federal rates for FY 2025, prior to adjustment for case-mix.
C. Case-Mix Adjustment
Under section 1888(e)(4)(G)(i) of the Act, the Federal rate also incorporates an adjustment to account for facility case-mix, using a classification system that accounts for the relative resource utilization of different patient types. The statute specifies that the adjustment is to reflect both a resident classification system that the Secretary establishes to account for the relative resource use of different patient types, as well as resident assessment data and other data that the Secretary considers appropriate. In the FY 2019 final rule (83 FR 39162, August 8, 2018), we finalized a new case-mix classification model, the PDPM, which took effect beginning October 1, 2019. The previous RUG-IV model classified most patients into a therapy payment group and primarily used the volume of therapy services provided to the patient as the basis for payment classification, thus creating an incentive for SNFs to furnish therapy regardless of the individual patient's unique characteristics, goals, or needs. PDPM eliminates this incentive and improves the overall accuracy and appropriateness of SNF payments by classifying patients into payment groups based on specific, data-driven patient characteristics, while simultaneously reducing the administrative burden on SNFs.
The PDPM uses clinical data from the MDS to assign case-mix classifiers to each patient that are then used to calculate a per diem payment under the SNF PPS, consistent with the provisions of section 1888(e)(4)(G)(i) of the Act. As outlined in section IV.A. of the proposed rule, the clinical orientation of the case-mix classification system supports the SNF PPS's use of an administrative presumption that considers a beneficiary's initial case-mix classification to assist in making certain SNF level of care determinations. Further, because the MDS is used as a basis for payment, as well as a clinical assessment, we have provided extensive training on proper coding and the timeframes for MDS completion in our Resident Assessment Instrument (RAI) Manual. As we have stated in prior rules, for an MDS to be considered valid for use in determining payment, the MDS assessment should be completed in compliance with the instructions in the RAI Manual in effect at the time the assessment is completed. For payment and quality monitoring purposes, the RAI Manual consists of both the Manual instructions and the interpretive guidance and policy clarifications posted on the appropriate MDS website at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/MDS30RAIManual.html.
Under section 1888(e)(4)(H) of the Act, each update of the payment rates must include the case-mix classification methodology applicable for the upcoming FY. The FY 2025 payment rates set forth in this final rule reflect the use of the PDPM case-mix classification system from October 1, 2023, through September 30, 2024. The case-mix adjusted PDPM payment rates for FY 2025 are listed separately for urban and rural SNFs, in Tables 5 and 6 with corresponding case-mix values.
Given the differences between the previous RUG-IV model and PDPM in terms of patient classification and billing, it was important that the format of Tables 5 and 6 reflect these differences. More specifically, under both RUG-IV and PDPM, providers use a Health Insurance Prospective Payment System (HIPPS) code on a claim to bill for covered SNF services. Under RUG-IV, the HIPPS code included the three-character RUG-IV group into which the patient classified, as well as a two-character assessment indicator code that represented the assessment used to generate this code. Under PDPM, while providers still use a HIPPS code, the characters in that code represent different things. For example, the first character represents the PT and OT group into which the patient classifies. If the patient is classified into the PT and OT group “TA”, then the first character in the patient's HIPPS code would be an A. Similarly, if the patient is classified into the SLP group “SB”, then the second character in the patient's HIPPS code would be a B. The third character represents the Nursing group into which the patient classifies. The fourth character represents the NTA group into which the patient classifies. Finally, the fifth character represents the assessment used to generate the HIPPS code.
Tables 5 and 6 reflect the PDPM's structure. Accordingly, Column 1 of Tables 5 and 6 represents the character in the HIPPS code associated with a given PDPM component. Columns 2 and 3 provide the case-mix index and associated case-mix adjusted component rate, respectively, for the relevant PT group. Columns 4 and 5 provide the case-mix index and associated case-mix adjusted component rate, respectively, for the relevant OT group. Columns 6 and 7 provide the case-mix index and associated case-mix adjusted component rate, respectively, for the relevant SLP group. Column 8 provides the nursing case-mix group (CMG) that is connected with a given PDPM HIPPS character. For example, if the patient qualified for the nursing group CBC1, then the third character in the patient's HIPPS code would be a “P.” Columns 9 and 10 provide the case-mix index and associated case-mix adjusted component rate, respectively, for the relevant nursing group. Finally, columns 11 and 12 provide the case-mix index and associated case-mix adjusted component rate, respectively, for the relevant NTA group.
Tables 5 and 6 do not reflect adjustments which may be made to the SNF PPS rates as a result of the SNF VBP Program, outlined in section VII. of this final rule, or other adjustments, such as the variable per diem adjustment.
D. Wage Index Adjustment
Section 1888(e)(4)(G)(ii) of the Act requires that we adjust the Federal rates to account for differences in area wage levels, using a wage index that the Secretary determines appropriate. Since the inception of the SNF PPS, we have used hospital inpatient wage data in developing a wage index to be applied to SNFs. We will continue this practice for FY 2025, as we continue to believe that in the absence of SNF-specific wage data, using the hospital inpatient wage index data is appropriate and reasonable for the SNF PPS. As explained in the update notice for FY 2005 (69 FR 45786), the SNF PPS does not use the hospital area wage index's occupational mix adjustment, as this adjustment serves specifically to define the occupational categories more clearly in a hospital setting; moreover, the collection of the occupational wage data under the inpatient prospective payment system (IPPS) also excludes any wage data related to SNFs. Therefore, we believe that using the updated wage data exclusive of the occupational mix adjustment continues to be appropriate for SNF payments. As in previous years, we continue to use the pre-reclassified IPPS hospital wage data, without applying the occupational mix, rural floor, or outmigration adjustment, as the basis for the SNF PPS wage index. For FY 2025, the updated wage data are for hospital cost reporting periods beginning on or after October 1, 2020, and before October 1, 2021 (FY 2021 cost report data).
We note that section 315 of the Medicare, Medicaid, and SCHIP Benefits Improvement and Protection Act of 2000 (BIPA) (Pub. L. 106-554, enacted December 21, 2000) gave the Secretary the discretion to establish a geographic reclassification procedure specific to SNFs, but only after collecting the data necessary to establish a SNF PPS wage index that is based on wage data from nursing homes. To date, this has proven to be unfeasible due to the volatility of existing SNF wage data and the significant amount of resources that would be required to improve the quality of the data. More specifically, auditing all SNF cost reports, similar to the process used to audit inpatient hospital cost reports for purposes of the IPPS wage index, would place a burden on providers in terms of recordkeeping and completion of the cost report worksheet. Adopting such an approach would require a significant commitment of resources by CMS and the Medicare Administrative Contractors (MACs), potentially far in excess of those required under the IPPS, given that there are nearly five times as many SNFs as there are inpatient hospitals. While we do not believe this undertaking is feasible at this time, we will continue to explore implementation of a spot audit process to improve SNF cost reports to ensure they are adequately accurate for cost development purposes, in such a manner as to permit us to establish a SNF-specific wage index in the future.
In addition, we will continue to use the same methodology discussed in the SNF PPS final rule for FY 2008 (72 FR 43423) to address those geographic areas in which there are no hospitals, and thus, no hospital wage index data on which to base the calculation of the FY 2025 SNF PPS wage index. For rural geographic areas that do not have hospitals and, therefore, lack hospital wage data on which to base an area wage adjustment, we will continue using the average wage index from all contiguous Core-Based Statistical Areas (CBSAs) as a reasonable proxy. For FY 2025, the only rural area without wage index data available is North Dakota. We have determined that the borders of 18 rural counties are local and contiguous with 8 urban counties. Therefore, under this methodology, the wage indexes for the counties of Burleigh/Morton/Oliver (CBSA 13900: 0.9020), Cass (CBSA 22020: 0.8763), Grand Forks (CBSA 24220: 0.7865), and McHenry/Renville/Ward (CBSA 33500: 0.7686) are averaged, resulting in an imputed rural wage index of 0.8334 for rural North Dakota for FY 2025. In past years for rural Puerto Rico, we did not apply this methodology due to the distinct economic circumstances there; due to the close proximity of almost all of Puerto Rico's various urban and non-urban areas, this methodology will produce a wage index for rural Puerto Rico that is higher than that in half of its urban areas. However, because rural Puerto Rico now has hospital wage index data on which to base an area wage adjustment, we will not apply this policy for FY 2025. For urban areas without specific hospital wage index data, we will continue using the average wage indexes of all urban areas within the State to serve as a reasonable proxy for the wage index of that urban CBSA. For FY 2025, the only urban area without wage index data available is CBSA 25980, Hinesville-Fort Stewart, GA.
In the SNF PPS final rule for FY 2006 (70 FR 45026, August 4, 2005), we adopted the changes discussed in OMB Bulletin No. 03-04 (June 6, 2003), which announced revised definitions for MSAs and the creation of micropolitan statistical areas and combined statistical areas. In adopting the CBSA geographic designations, we provided for a 1-year transition in FY 2006 with a blended wage index for all providers. For FY 2006, the wage index for each provider consisted of a blend of 50 percent of the FY 2006 MSA-based wage index and 50 percent of the FY 2006 CBSA-based wage index (both using FY 2002 hospital data). We referred to the blended wage index as the FY 2006 SNF PPS transition wage index. As discussed in the SNF PPS final rule for FY 2006 (70 FR 45041), after the expiration of this 1-year transition on September 30, 2006, we used the full CBSA-based wage index values.
In the FY 2015 SNF PPS final rule (79 FR 45644 through 45646), we finalized changes to the SNF PPS wage index based on the newest OMB delineations, as described in OMB Bulletin No. 13-01, beginning in FY 2015, including a 1-year transition with a blended wage index for FY 2015. OMB Bulletin No. 13-01 established revised delineations for Metropolitan Statistical Areas, Micropolitan Statistical Areas, and Combined Statistical Areas in the United States and Puerto Rico based on the 2010 Census and provided guidance on the use of the delineations of these statistical areas using standards published in the June 28, 2010 Federal Register (75 FR 37246 through 37252). Subsequently, on July 15, 2015, OMB issued OMB Bulletin No. 15-01, which provided minor updates to and superseded OMB Bulletin No. 13-01 that was issued on February 28, 2013. The attachment to OMB Bulletin No. 15-01 provided detailed information on the update to statistical areas since February 28, 2013. The updates provided in OMB Bulletin No. 15-01 were based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to Census Bureau population estimates for July 1, 2012, and July 1, 2013, and were adopted under the SNF PPS in the FY 2017 SNF PPS final rule (81 FR 51983, August 5, 2016). In addition, on August 15, 2017, OMB issued Bulletin No. 17-01 which announced a new urban CBSA, Twin Falls, Idaho (CBSA 46300), which was adopted in the SNF PPS final rule for FY 2019 (83 FR 39173, August 8, 2018).
As discussed in the FY 2021 SNF PPS final rule (85 FR 47594), we adopted the revised OMB delineations identified in OMB Bulletin No. 18-04 (available at https://www.whitehouse.gov/wp-content/uploads/2018/09/Bulletin-18-04.pdf) beginning October 1, 2020, including a 1-year transition for FY 2021 under which we applied a 5 percent cap on any decrease in a hospital's wage index compared to its wage index for the prior fiscal year (FY 2020). The updated OMB delineations more accurately reflect the contemporary urban and rural nature of areas across the country, and the use of such delineations allows us to determine more accurately the appropriate wage index and rate tables to apply under the SNF PPS.
In the FY 2023 SNF PPS final rule (87 FR 47521 through 47525), we finalized a policy to apply a permanent 5 percent cap on any decreases to a provider's wage index from its wage index in the prior year, regardless of the circumstances causing the decline. We amended the SNF PPS regulations at 42 CFR 413.337(b)(4)(ii) to reflect this permanent cap on wage index decreases. Additionally, we finalized a policy that a new SNF would be paid the wage index for the area in which it is geographically located for its first full or partial FY with no cap applied because a new SNF would not have a wage index in the prior FY. A full discussion of the adoption of this policy is found in the FY 2023 SNF PPS final rule.
As we previously stated in the FY 2008 SNF PPS proposed and final rules (72 FR 25538 through 25539, and 72 FR 43423), this and all subsequent SNF PPS rules and notices are considered to incorporate any updates and revisions set forth in the most recent OMB bulletin that applies to the hospital wage data used to determine the current SNF PPS wage index. OMB issued further revised CBSA delineations in OMB Bulletin No. 20-01, on March 6, 2020 (available on the web at https://www.whitehouse.gov/wp-content/uploads/2020/03/Bulletin-20-01.pdf). However, we determined that the changes in OMB Bulletin No. 20-01 do not impact the CBSA-based labor market area delineations adopted in FY 2021. Therefore, we did not propose to adopt the revised OMB delineations identified in OMB Bulletin No. 20-01 for FY 2022 through FY 2024.
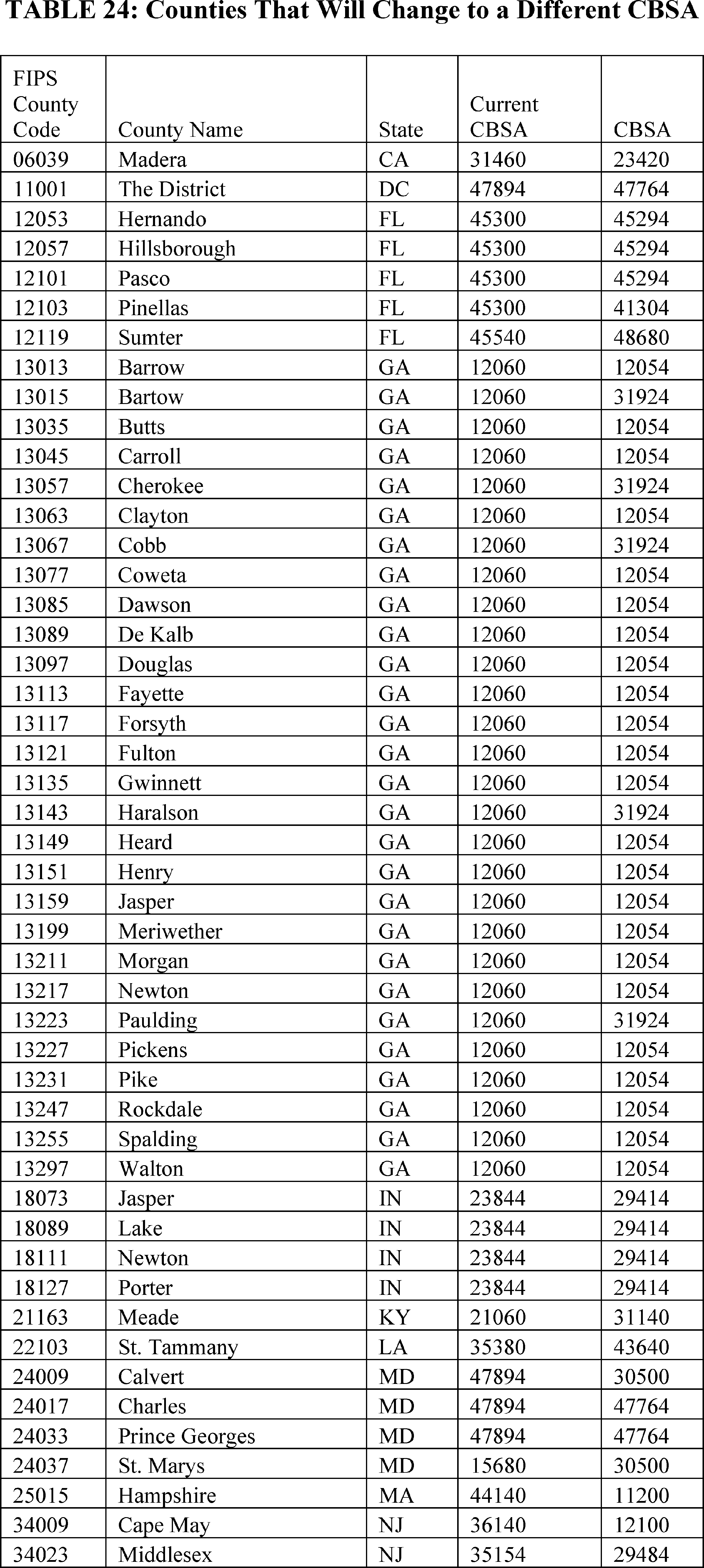
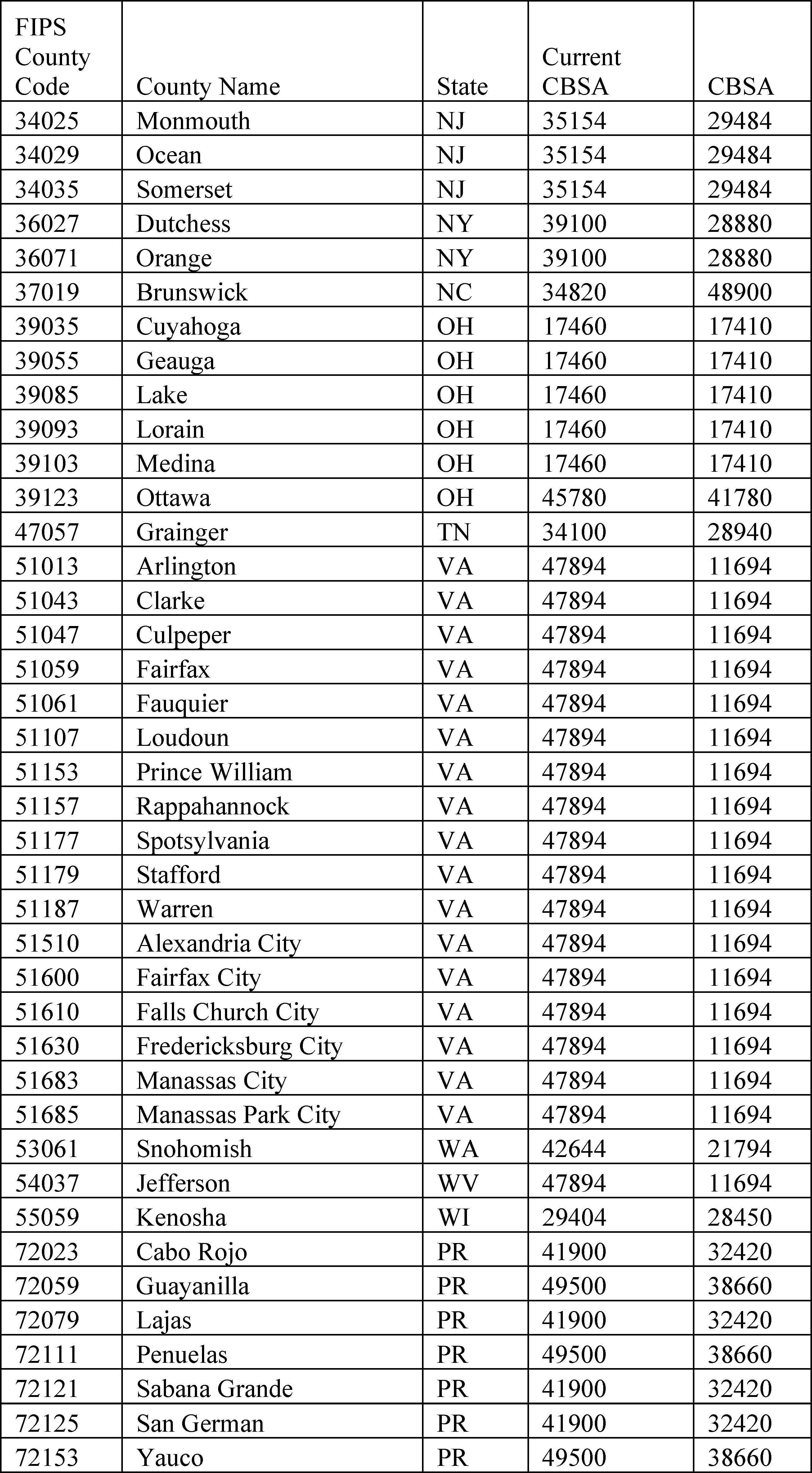
On July 21, 2023, OMB issued OMB Bulletin No. 23-01 which updates and supersedes OMB Bulletin No. 20-01 based on the decennial census. OMB Bulletin No. 23-01 revised delineations for CBSAs which are made up of counties and equivalent entities (for example, boroughs, a city and borough, and a municipality in Alaska, planning regions in Connecticut, parishes in Louisiana, municipios in Puerto Rico, and independent cities in Maryland, Missouri, Nevada, and Virginia). For FY 2025, we proposed to adopt the revised OMB delineations identified in OMB Bulletin No. 23-01 (available at https://www.whitehouse.gov/wp-content/uploads/2023/07/OMB-Bulletin-23-01.pdf). The wage index applicable to FY 2025 is set forth in Table A and B, available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/WageIndex.html.
Once calculated, we will apply the wage index adjustment to the labor-related portion of the Federal rate. Each year, we calculate a labor-related share, based on the relative importance of labor-related cost categories (that is, those cost categories that are labor-intensive and vary with the local labor market) in the input price index. In the SNF PPS final rule for FY 2022 (86 FR 42437), we finalized a proposal to revise the labor-related share to reflect the relative importance of the 2018-based SNF market basket cost weights for the following cost categories: Wages and Salaries; Employee Benefits; Professional Fees: Labor-Related; Administrative and Facilities Support Services; Installation, Maintenance, and Repair Services; All Other: Labor-Related Services; and a proportion of Capital-Related expenses. The methodology for calculating the labor-related portion beginning in FY 2022 is discussed in detail in the FY 2022 SNF PPS final rule (86 FR 42461 through 42463). Effective beginning in FY 2025, as described in section VI.A. of this final rule, we are rebasing and revising the labor-related share to reflect the relative importance of the 2022-based SNF market basket cost weights for the following categories: Wages and Salaries; Employee Benefits; Professional Fees: Labor-Related; Administrative and Facilities Support Services; Installation, Maintenance, and Repair Services; All Other: Labor-Related Services; and a proportion of Capital-Related expenses. The methodology for calculating the labor-related share of the 2022-based SNF market basket is detailed in section VI.A.4. of this final rule.
We calculate the labor-related relative importance from the SNF market basket, and it approximates the labor-related portion of the total costs after taking into account historical and projected price changes between the base year and FY 2025. The price proxies that move the different cost categories in the market basket do not necessarily change at the same rate, and the relative importance captures these changes. Accordingly, the relative importance figure more closely reflects the cost share weights for FY 2025 than the base year weights from the SNF market basket. We calculate the labor-related relative importance for FY 2025 in four steps. First, we compute the FY 2025 price index level for the total market basket and each cost category of the market basket. Second, we calculate a ratio for each cost category by dividing the FY 2025 price index level for that cost category by the total market basket price index level. Third, we determine the FY 2025 relative importance for each cost category by multiplying this ratio by the base year (2022) weight. Finally, we add the FY 2025 relative importance for each of the labor-related cost categories (Wages and Salaries; Employee Benefits; Professional Fees: Labor-Related; Administrative and Facilities Support Services; Installation, Maintenance, and Repair Services; All Other: Labor-Related Services; and a portion of Capital-Related expenses) to produce the FY 2025 labor-related relative importance.
For the proposed rule, the labor-related share for FY 2025 was based on IGI's fourth quarter 2023 forecast of the proposed 2022-based SNF market basket with historical data through third-quarter 2023. For this final rule, as proposed, we estimate the labor-related share for FY 2025 based on IGI's more recent second quarter 2024 forecast, with historical data through the first quarter of 2024. Table 7 summarizes the labor-related share for FY 2025, based on IGI's second quarter 2024 forecast of the 2022-based SNF market basket, compared to the labor-related share that was used for the FY 2024 SNF PPS final rule.
To calculate the labor portion of the case-mix adjusted per diem rate, we will multiply the total case-mix adjusted per diem rate, which is the sum of all five case-mix adjusted components into which a patient classifies, and the non-case-mix component rate, by the FY 2025 labor-related share percentage provided in Table 7. The remaining portion of the rate will be the non-labor portion. Under the previous RUG-IV model, we included tables which provided the case-mix adjusted RUG-IV rates, by RUG-IV group, broken out by total rate, labor portion and non-labor portion, such as Table 9 of the FY 2019 SNF PPS final rule (83 FR 39175). However, as we discussed in the FY 2020 final rule (84 FR 38738), under PDPM, as the total rate is calculated as a combination of six different component rates, five of which are case-mix adjusted, and given the sheer volume of possible combinations of these five case-mix adjusted components, it is not feasible to provide tables similar to those that existed in the prior rulemaking.
Therefore, to aid interested parties in understanding the effect of the wage index on the calculation of the SNF per diem rate, we have included a hypothetical rate calculation in Table 9.
Section 1888(e)(4)(G)(ii) of the Act also requires that we apply this wage index in a manner that does not result in aggregate payments under the SNF PPS that are greater or less than would otherwise be made if the wage adjustment had not been made. For FY 2025 (Federal rates effective October 1, 2023), we apply an adjustment to fulfill the budget neutrality requirement. We meet this requirement by multiplying each of the components of the unadjusted Federal rates by a budget neutrality factor, equal to the ratio of the weighted average wage adjustment factor for FY 2025 to the weighted average wage adjustment factor for FY 2025. For this calculation, we will use the same FY 2023 claims utilization data for both the numerator and denominator of this ratio. We define the wage adjustment factor used in this calculation as the labor portion of the rate component multiplied by the wage index plus the non-labor portion of the rate component. The budget neutrality factor for FY 2025 is 1.0005.
In the proposed rule, we noted that if more recent data became available (for example, revised wage data), we would use such data, if appropriate, to determine the wage index budget neutrality factor in the SNF PPS final rule.
E. SNF Value-Based Purchasing Program
Beginning with payment for services furnished on October 1, 2018, section 1888(h) of the Act requires the Secretary to reduce the adjusted Federal per diem rate determined under section 1888(e)(4)(G) of the Act otherwise applicable to a SNF for services furnished during a fiscal year by 2 percent, and to adjust the resulting rate for a SNF by the value-based incentive payment amount earned by the SNF based on the SNF's performance score for that fiscal year under the SNF VBP Program. To implement these requirements, we finalized in the FY 2019 SNF PPS final rule the addition of § 413.337(f) to our regulations (83 FR 39178).
Please see section VIII. of this final rule for further discussion of the updates we are finalizing for the SNF VBP Program.
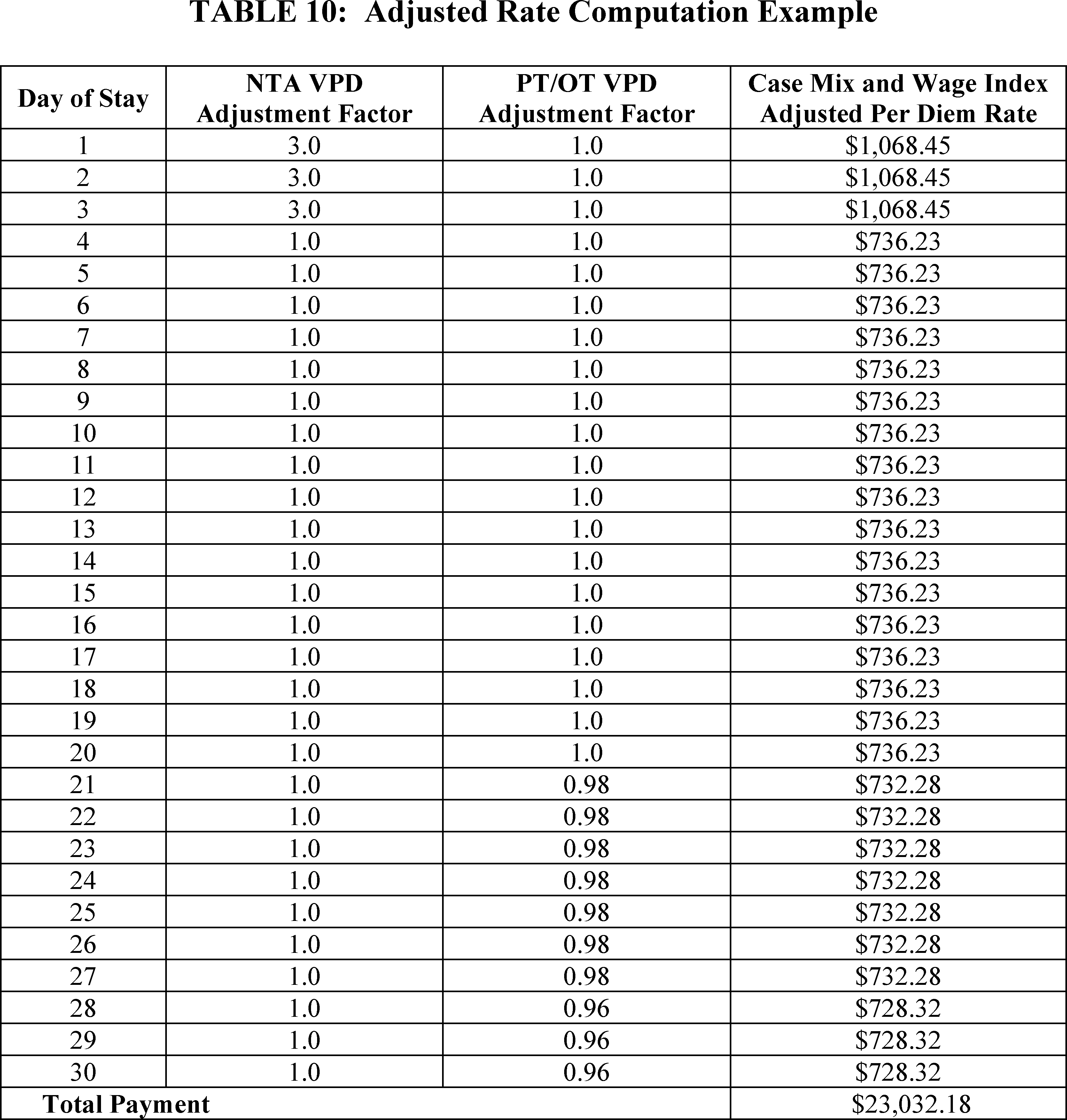
F. Adjusted Rate Computation Example
Tables 8 through 10 provide examples generally illustrating payment calculations during FY 2025 under PDPM for a hypothetical 30-day SNF stay, involving the hypothetical SNF XYZ, located in Frederick, MD (Urban CBSA 23224), for a hypothetical patient who is classified into such groups that the patient's HIPPS code is NHNC1. Table 8 shows the adjustments made to the Federal per diem rates (prior to application of any adjustments under the SNF VBP Program as discussed) to compute the provider's case-mix adjusted per diem rate for FY 2025, based on the patient's PDPM classification, as well as how the variable per diem (VPD) adjustment factor affects calculation of the per diem rate for a given day of the stay. Table 9 shows the adjustments made to the case-mix adjusted per diem rate from Table 8 to account for the provider's wage index. The wage index used in this example is based on the FY 2025 SNF PPS wage index that appears in Table A available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/WageIndex.html. Finally, Table 10 provides the case-mix and wage index adjusted per-diem rate for this patient for each day of the 30-day stay, as well as the total payment for this stay. Table 10 also includes the VPD adjustment factors for each day of the patient's stay, to clarify why the patient's per diem rate changes for certain days of the stay. As illustrated in Table 10, SNF XYZ's total PPS payment for this particular patient's stay would equal $23,032.18.
V. Additional Aspects of the SNF PPS
A. SNF Level of Care—Administrative Presumption
The establishment of the SNF PPS did not change Medicare's fundamental requirements for SNF coverage. However, because the case-mix classification is based, in part, on the beneficiary's need for skilled nursing care and therapy, we have attempted, where possible, to coordinate claims review procedures with the existing resident assessment process and case-mix classification system outlined in section III.C. of the proposed rule. This approach includes an administrative presumption that utilizes a beneficiary's correct assignment, at the outset of the SNF stay, of one of the case-mix classifiers designated for this purpose to assist in making certain SNF level of care determinations.
In accordance with § 413.345, we include in each update of the Federal payment rates in the Federal Register a discussion of the resident classification system that provides the basis for case-mix adjustment. We also designate those specific classifiers under the case-mix classification system that represent the required SNF level of care, as provided in 42 CFR 409.30. This designation reflects an administrative presumption that those beneficiaries who are correctly assigned one of the designated case-mix classifiers on the initial Medicare assessment are automatically classified as meeting the SNF level of care definition up to and including the assessment reference date (ARD) for that assessment.
A beneficiary who does not qualify for the presumption is not automatically classified as either meeting or not meeting the level of care definition, but instead receives an individual determination on this point using the existing administrative criteria. This presumption recognizes the strong likelihood that those beneficiaries who are correctly assigned one of the designated case-mix classifiers during the immediate post-hospital period would require a covered level of care, which would be less likely for other beneficiaries.
In the July 30, 1999 final rule (64 FR 41670), we indicated that we would announce any changes to the guidelines for Medicare level of care determinations related to modifications in the case-mix classification structure. The FY 2018 final rule (82 FR 36544) further specified that we would henceforth disseminate the standard description of the administrative presumption's designated groups via the SNF PPS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/ index.html (where such designations appear in the paragraph entitled “Case Mix Adjustment”) and would publish such designations in rulemaking only to the extent that we actually intend to propose changes in them. Under that approach, the set of case-mix classifiers designated for this purpose under PDPM was finalized in the FY 2019 SNF PPS final rule (83 FR 39253) and is posted on the SNF PPS website ( https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/index.html), in the paragraph entitled “Case Mix Adjustment.”
However, we note that this administrative presumption policy does not supersede the SNF's responsibility to ensure that its decisions relating to level of care are appropriate and timely, including a review to confirm that any services prompting the assignment of one of the designated case-mix classifiers (which, in turn, serves to trigger the administrative presumption) are themselves medically necessary. As we explained in the FY 2000 SNF PPS final rule (64 FR 41667), the administrative presumption is itself rebuttable in those individual cases in which the services actually received by the resident do not meet the basic statutory criterion of being reasonable and necessary to diagnose or treat a beneficiary's condition (according to section 1862(a)(1) of the Act). Accordingly, the presumption would not apply, for example, in those situations where the sole classifier that triggers the presumption is itself assigned through the receipt of services that are subsequently determined to be not reasonable and necessary. Moreover, we want to stress the importance of careful monitoring for changes in each patient's condition to determine the continuing need for Part A SNF benefits after the Assessment Reference Date (ARD) of the initial Medicare assessment.
B. Consolidated Billing
Sections 1842(b)(6)(E) and 1862(a)(18) of the Act (as added by section 4432(b) of the BBA 1997) require a SNF to submit consolidated Medicare bills to its Medicare Administrative Contractor (MAC) for almost all of the services that its residents receive during the course of a covered Part A stay. In addition, section 1862(a)(18) of the Act places the responsibility with the SNF for billing Medicare for physical therapy, occupational therapy, and speech-language pathology services that the resident receives during a noncovered stay. Section 1888(e)(2)(A) of the Act excludes a small list of services from the consolidated billing provision (primarily those services furnished by physicians and certain other types of practitioners), which remain separately billable under Part B when furnished to a SNF's Part A resident. These excluded service categories are discussed in greater detail in section V.B.2. of the May 12, 1998 interim final rule (63 FR 26295 through 26297). Effective with services furnished on or after January 1, 2024, section 4121(a)(4) of the Consolidated Appropriations Act, 2023 (CAA, 2023) (Pub. L. 117-328, enacted December 29, 2022) added marriage and family therapists and mental health counselors to the list of practitioners at section 1888(e)(2)(A)(ii) of the Act whose services are excluded from the consolidated billing provision.
Section 103 of the Medicare, Medicaid, and SCHIP Balanced Budget Refinement Act of 1999 (BBRA 1999) (Pub. L. 106-113, enacted November 29, 1999) amended section 1888(e)(2)(A)(iii) of the Act by further excluding a number of individual high-cost, low probability services, identified by Healthcare Common Procedure Coding System (HCPCS) codes, within several broader categories (chemotherapy items, chemotherapy administration services, radioisotope services, and customized prosthetic devices) that otherwise remained subject to the provision. We discuss this BBRA 1999 amendment in greater detail in the SNF PPS proposed and final rules for FY 2001 (65 FR 19231 through 19232, April 10, 2000, and 65 FR 46790 through 46795, July 31, 2000), as well as in Program Memorandum AB-00-18 (Change Request #1070), issued March 2000, which is available online at www.cms.gov/transmittals/downloads/ab001860.pdf.
As explained in the FY 2001 proposed rule (65 FR 19232), the amendments enacted in section 103 of the BBRA 1999 not only identified for exclusion from this provision a number of particular service codes within four specified categories (that is, chemotherapy items, chemotherapy administration services, radioisotope services, and customized prosthetic devices), but also gave the Secretary the authority to designate additional, individual services for exclusion within each of these four specified service categories. In the proposed rule for FY 2001, we also noted that the BBRA 1999 Conference report (H.R. Conf. Rep. No. 106-479 at 854 (1999)) characterizes the individual services that this legislation targets for exclusion as high-cost, low probability events that could have devastating financial impacts because their costs far exceed the payment SNFs receive under the PPS. According to the conferees, section 103(a) of the BBRA 1999 is an attempt to exclude from the PPS certain services and costly items that are provided infrequently in SNFs. By contrast, the amendments enacted in section 103 of the BBRA 1999 do not designate for exclusion any of the remaining services within those four categories (thus, leaving all of those services subject to SNF consolidated billing), because they are relatively inexpensive and are furnished routinely in SNFs.
Effective with items and services furnished on or after October 1, 2021, section 134 in Division CC of the CAA, 2021 established an additional fifth category of excluded codes in section 1888(e)(2)(A)(iii)(VI) of the Act, for certain blood clotting factors for the treatment of patients with hemophilia and other bleeding disorders along with items and services related to the furnishing of such factors under section 1842(o)(5)(C) of the Act. Like the provisions enacted in the BBRA 1999, section 1888(e)(2)(A)(iii)(VI) of the Act gives the Secretary the authority to designate additional items and services for exclusion within the category of items and services related to blood clotting factors, as described in that section.
A detailed discussion of the legislative history of the consolidated billing provision is available on the SNF PPS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/Downloads/Legislative_History_2018-10-01.pdf.
As we further explained in the final rule for FY 2001 (65 FR 46790), and as is consistent with our longstanding policy, any additional service codes that we might designate for exclusion under our discretionary authority must meet the same statutory criteria used in identifying the original codes excluded from consolidated billing under section 103(a) of the BBRA 1999: they must fall within one of the five service categories specified in the BBRA 1999 and CAA, 2021; and they also must meet the same standards of high cost and low probability in the SNF setting, as discussed in the BBRA 1999 Conference report. Accordingly, we characterized this statutory authority to identify additional service codes for exclusion as essentially affording the flexibility to revise the list of excluded codes in response to changes of major significance that may occur over time (for example, the development of new medical technologies or other advances in the state of medical practice) (65 FR 46791).
In the proposed rule, we specifically solicited public comments identifying HCPCS codes in any of these five service categories (chemotherapy items, chemotherapy administration services, radioisotope services, customized prosthetic devices, and blood clotting factors) representing recent medical advances that might meet our criteria for exclusion from SNF consolidated billing. We considered excluding a particular service if it met our criteria for exclusion as specified previously in this section of the preamble. We requested that commenters identify in their comments the specific HCPCS code that is associated with the service in question, as well as their rationale for requesting that the identified HCPCS code(s) be excluded.
We noted that the original BBRA amendment and the CAA, 2021 identified a set of excluded items and services by means of specifying individual HCPCS codes within the designated categories that were in effect as of a particular date (in the case of the BBRA 1999, July 1, 1999, and in the case of the CAA, 2021, July 1, 2020), as subsequently modified by the Secretary. In addition, as noted previously in this section of the preamble, the statute (sections 1888(e)(2)(A)(iii)(II) through (VI) of the Act) gives the Secretary authority to identify additional items and services for exclusion within the five specified categories of items and services described in the statute, which are also designated by HCPCS code. Designating the excluded services in this manner makes it possible for us to utilize program issuances as the vehicle for accomplishing routine updates to the excluded codes to reflect any minor revisions that might subsequently occur in the coding system itself, such as the assignment of a different code number to a service already designated as excluded, or the creation of a new code for a type of service that falls within one of the established exclusion categories and meets our criteria for exclusion.
Accordingly, we stated in the proposed rule that if we identify through the current rulemaking cycle any new services that meet the criteria for exclusion from SNF consolidated billing, we will identify these additional excluded services by means of the HCPCS codes that are in effect as of a specific date (in this case, October 1, 2024). By making any new exclusions in this manner, we can similarly accomplish routine future updates of these additional codes through the issuance of program instructions. The latest list of excluded codes can be found on the SNF Consolidated Billing website at https://www.cms.gov/Medicare/Billing/SNFConsolidatedBilling.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: A few commenters suggested CMS consider several items for exclusion from SNF consolidated billing which have already been suggested and considered in previous rulemaking, including: Imatinib; Erleada; Venetoclax; Dasatinib; Ponatinib; Cabozantinib; Sunitinib; Lenalidomide; and Lupron (leuprolide).
Response: We have considered each of these suggestions in previous rulemaking and we reiterate that these items cannot be excluded from SNF consolidated billing. We refer commenters to previous SNF final rules in which these suggestions were addressed, including FY 2024 (88 FR 53200, August 7, 2023) and FY 2021 (85 FR 47609 through 47610, August 5, 2020).
Comment: Commenters suggested several specific HCPCS codes for exclusion that have not already been addressed in previous rulemaking: Jakafi (ruxolitinib), Tafinlar (dabrafenib), Nilotinib, and Tumor Treating Fields (“TTFields”) therapy.
Response: With regard to Jakafi, Tafinlar, and Nilotinib, these three services are all targeted medications that “target” specific signals involved in cancer growth, but they are not chemotherapy treatments. Chemotherapy is a specific subset of cancer treatment characterized by its systemic attacking of cell growth. Likewise, Tumor Treating Fields therapy is a type of electromagnetic field therapy used to treat cancer and is not a form of chemotherapy. As these are not considered chemotherapy services, the suggestions do not fit the chemotherapy category or any other of the five service categories in which we have statutory authority to add exclusions, and therefore we may not exclude these items from SNF consolidated billing. Excluding such items would require an act of Congress to modify the law.
Comment: Commenters reiterated several general comments that are outside of the agency's statutory authority and/or have already been addressed in prior rulemaking cycles. Comments stated that CMS should modify consolidated billing rules for SNFs to use a “price/cost threshold” rather than base the program on specific HCPCS codes. Comments requested CMS exclude non-chemotherapy cancer treatments. Another comment requested the exclusion of HIV drugs and associated administration and other less commonly used medication and administration drugs and treatments that exceed SNF reimbursement rates.
Response: As previously specified in this section of the preamble, the authority afforded to us under the law to modify the list of services excluded from SNF consolidated billing is limited to adding or removing HCPCS codes representing high-cost low-probability services from the five specific service categories identified in the statute. Any of the modifications to consolidated billing and/or the SNF program suggested by the previously mentioned comments would require an act of Congress to modify the law.
Comment: A commenter requested that CMS consider adopting a formalized process in which entities may propose an item, service, or drug be added to the excluded list for consolidated billing on a case-by-case or permanent basis.
Response: In addition to conducting our own routine internal reviews of new and modified HCPCS codes, we solicit feedback from interested parties on consolidated billing exclusions through this annual rulemaking process. At this time, we consider this process sufficient to identify services that should be excluded.
Comment: Commenters stated general appreciation for CMS soliciting public comments to identify HCPCS codes that meet the criteria for exclusion from consolidated billing. Comments stated they would continue to try to identify such HCPCS codes.
Response: We thank commenters for their review.
C. Payment for SNF-Level Swing-Bed Services
Section 1883 of the Act permits certain small, rural hospitals to enter into a Medicare swing-bed agreement, under which the hospital can use its beds to provide either acute- or SNF-level care, as needed. For critical access hospitals (CAHs), Part A pays on a reasonable cost basis for SNF-level services furnished under a swing-bed agreement. However, in accordance with section 1888(e)(7) of the Act, SNF-level services furnished by non-CAH rural hospitals are paid under the SNF PPS, effective with cost reporting periods beginning on or after July 1, 2002. As explained in the FY 2002 final rule (66 FR 39562), this effective date is consistent with the statutory provision to integrate swing-bed rural hospitals into the SNF PPS by the end of the transition period, June 30, 2002.
Accordingly, all non-CAH swing-bed rural hospitals have now come under the SNF PPS. Therefore, all rates and wage indexes outlined in earlier sections of this proposed rule for the SNF PPS also apply to all non-CAH swing-bed rural hospitals. As finalized in the FY 2010 SNF PPS final rule (74 FR 40356 through 40357), effective October 1, 2010, non-CAH swing-bed rural hospitals are required to complete an MDS 3.0 swing-bed assessment which is limited to the required demographic, payment, and quality items. As discussed in the FY 2019 SNF PPS final rule (83 FR 39235), revisions were made to the swing bed assessment to support implementation of PDPM, effective October 1, 2019. A discussion of the assessment schedule and the MDS effective beginning FY 2020 appears in the FY 2019 SNF PPS final rule (83 FR 39229 through 39237). The latest changes in the MDS for swing-bed rural hospitals appear on the SNF PPS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/index.html.
VI. Other SNF PPS Issues
A. Rebasing and Revising the SNF Market Basket
Section 1888(e)(5)(A) of the Act requires the Secretary to establish a market basket that reflects the changes over time in the prices of an appropriate mix of goods and services included in covered SNF services. Accordingly, we have developed a SNF market basket that encompasses the most commonly used cost categories for SNF routine services, ancillary services, and capital-related expenses.
The SNF market basket is used to compute the market basket percentage increase that is used to update the SNF Federal per diem rates on an annual basis, as required by section 1888(e)(4)(E)(ii)(IV) of the Act. This market basket percentage increase is adjusted by a forecast error adjustment, if applicable, and then further adjusted by the application of a productivity adjustment as required by section 1888(e)(5)(B)(ii) of the Act and described in section III.B.4. of the proposed rule. The SNF market basket is also used to determine the labor-related share on an annual basis.
The SNF market basket is a fixed-weight, Laspeyres-type price index. A Laspeyres price index measures the change in price, over time, of the same mix of goods and services purchased in the base period. Any changes in the quantity or mix of goods and services (that is, intensity) purchased over time relative to a base period are not measured.
The index itself is constructed in three steps. First, a base period is selected (the base period is 2022) and total base period costs are estimated for a set of mutually exclusive and exhaustive spending categories and the proportion of total costs that each category represents is calculated. These proportions are called cost weights. Second, each cost category is matched to an appropriate price or wage variable, referred to as a price proxy. In nearly every instance, these price proxies are derived from publicly available statistical series that are published on a consistent schedule (preferably at least on a quarterly basis). Finally, the cost weight for each cost category is multiplied by the level of its respective price proxy. The sum of these products (that is, the cost weights multiplied by their price levels) for all cost categories yields the composite index level of the market basket in a given period. Repeating this step for other periods produces a series of market basket levels over time. Dividing an index level for a given period by an index level for an earlier period produces a rate of growth in the input price index over that timeframe.
Since the inception of the SNF PPS, the market basket used to update SNF PPS payments has been periodically rebased and revised. We last rebased and revised the market basket applicable to the SNF PPS in the FY 2022 SNF PPS final rule (86 FR 42444 through 42463) where we adopted a 2018-based SNF market basket. References to the historical market baskets used to update SNF PPS payments are listed in the FY 2022 SNF PPS final rule (86 FR 42445).
Effective for FY 2025 and subsequent fiscal years, we proposed to rebase and revise the market basket to reflect 2022 Medicare-allowable total cost data (routine, ancillary, and capital-related) from freestanding SNFs and to revise applicable cost categories and price proxies used to determine the market basket. Medicare-allowable costs are those costs that are eligible to be paid under the SNF PPS. For example, the SNF market basket excludes home health agency (HHA) costs as these costs would be paid under the HHA PPS, and therefore, these costs are not SNF PPS Medicare-allowable costs. We proposed to maintain our policy of using data from freestanding SNFs, of which about 91 percent of SNFs that submitted a Medicare cost report for 2022 are represented in our sample shown in Table 11. We believe using freestanding SNF Medicare cost report data, as opposed to the hospital-based SNF Medicare cost report data, for the cost weight calculation is most appropriate because of the complexity of hospital-based data and the representativeness of the freestanding data. Because hospital-based SNF expenses are embedded in the hospital cost report, any attempt to incorporate data from hospital-based facilities requires more complex calculations and assumptions regarding the ancillary costs related to the hospital-based SNF unit. We believe the use of freestanding SNF cost report data is technically appropriate for reflecting the cost structures of SNFs serving Medicare beneficiaries.
We proposed to use 2022 as the base year as we believe that the 2022 Medicare cost reports represent the most recent, complete set of Medicare cost report data available to develop cost weights for SNFs at the time of rulemaking. We believe it is important to regularly rebase and revise the SNF market basket to reflect more recent data. Historically, the cost weights change minimally from year to year as they represent the percent of total costs rather than cost levels; however, given the COVID-19 Public Health Emergency (PHE), we have been monitoring the Medicare cost report data to see if a more frequent rebasing schedule is necessary than our recent historical precedent of about every 4 years. Accordingly, while it has been only three years since the last SNF rebasing, we proposed to incorporate data that is more reflective of recent SNF expenses that have been impacted over the most recent few years. The 2022 Medicare cost reports are for cost reporting periods beginning on and after October 1, 2021 and before October 1, 2022. While these dates appear to reflect fiscal year data, we noted in the proposed rule that a Medicare cost report that begins in this timeframe is generally classified as a “2022 cost report”. For example, we found that of the available 2022 Medicare cost reports for SNFs, approximately 7 percent had an October 1, 2021, begin date, approximately 75 percent of the reports had a January 1, 2022, begin date, and approximately 12 percent had a July 1, 2022 begin date. For this reason, we are defining the base year of the market basket as “2022-based” instead of “FY 2022-based”.
We received approximately 22 comments on the proposed rebasing and revising of the SNF market basket. A discussion of these comments, with our responses, appears throughout this section.
Comment: Several commenters noted that they support CMS' decision to rebase the SNF market basket 1 year earlier than is typical, and that rebasing and revising the market basket more frequently than the recent historical precedent of approximately every 4 years is warranted to more accurately reflect costs faced by SNFs at this time.
Response: We thank the commenters for their support in rebasing and revising of the SNF market basket, and we will continue to monitor the data that inform the frequency of the rebasing.
Comment: One commenter stated that the need for both auditing cost reports and requiring SNFs to submit audited cost reports is especially critical this year as CMS plans to rebase the SNF market basket using cost report data from 2022. They stated that there are too many indications of flawed and possibly fraudulent data, and CMS cannot simply assume that cost report data are accurate.
Response: We recognize the commenter's concerns and reiterate that accurate and complete reporting of all data on the Medicare cost reports by SNFs help to ensure that the cost weights for the SNF market basket are reflective of the cost structure of SNFs. We also note that we analyze the Medicare cost report data to evaluate their representativeness; for example, we reweight the data reported by ownership type and urban/rural so that it reflects the universe of providers and compare it to the proposed cost weights that are based on reported data. Our analysis shows the proposed cost weights are representative across these dimensions. In addition, we also trim the data to eliminate outliers as described in section VI.A.1.a of this final rule.
As stated in the FY 2024 SNF PPS final rule (88 FR 53212), auditing all SNF cost reports, similar to the process used to audit inpatient hospital cost reports for purposes of the IPPS wage index, would place a burden on providers in terms of recordkeeping and completion of the cost report worksheet. Adopting such an approach would require a significant commitment of resources by CMS and the Medicare Administrative Contractors (MACs), potentially far in excess of those required under the IPPS, given that there are nearly five times as many SNFs as there are IPPS hospitals. We continue to believe that the development of such an audit process could improve SNF cost reports, but we do not believe this undertaking is feasible at this time.
Final Decision: We are finalizing our proposal to rebase the SNF market basket to reflect a 2022 base year for FY 2025.
We provide a summary of the more detailed public comments received on our proposed methodology for developing the 2022-based SNF market basket and our responses in the sections that follow.
We proposed to develop cost category weights for the proposed 2022-based SNF market basket in two stages. The major types of costs underlying the proposed 2022-based SNF market basket are derived from the 2022 Medicare cost report data (CMS Form 2540-10, OMB NO. 0938-0463) for freestanding SNFs. Specifically, we used the Medicare cost reports for seven specific costs: Wages and Salaries; Employee Benefits; Contract Labor; Pharmaceuticals; Professional Liability Insurance; Home Office/Related Organization Contract Labor; and Capital-related. A residual “All Other” category is then estimated and reflects all remaining costs that are not captured in the seven types of costs identified above. The 2018-based SNF market basket similarly used 2018 Medicare cost report data. Second, we proposed to divide the residual “All Other” cost category into more detailed subcategories, using U.S. Department of Commerce Bureau of Economic Analysis' (BEA) 2017 Benchmark Input-Output (I-O) “The Use Table (Supply-Use Framework)” for the Nursing and Community Care Facilities industry (North American Industry Classification System (NAICS) code 623A00) aged to 2022 using applicable price proxy growth for each category of costs. Furthermore, we proposed to continue to use the same overall methodology as was used for the 2018-based SNF market basket to develop the capital related cost weights of the proposed 2022-based SNF market basket.
1. Development of Cost Categories and Weights
a. Use of Medicare Cost Report Data To Develop Major Cost Weights
In order to create a market basket that is representative of freestanding SNF providers serving Medicare patients and to help ensure accurate major cost weights (which is the percent of total Medicare-allowable costs, as defined below), we proposed to apply edits to remove reporting errors and outliers. Specifically, the SNF Medicare cost reports used to calculate the market basket cost weights exclude any providers that reported costs less than or equal to zero for the following categories: total facility costs (Worksheet B, part 1, column 18, line 100); total operating costs (Worksheet B, part 1, column 18, line 100 less Worksheet B, part 2, column 18, line 100); Medicare general inpatient routine service costs (Worksheet D, part 1, column 1, line 1); and Medicare PPS payments (Worksheet E, part 3, column 1, line 1). We also limited our sample to providers that had a Medicare cost report reporting period that was between 10 and 14 months. The final sample used included roughly 13,100 Medicare cost reports (about 90 percent of the universe of SNF Medicare cost reports for 2022). The sample of providers is representative of the national universe of providers by region (each region is represented within plus or minus 1 percentage point of universe distribution), by ownership-type (proprietary, nonprofit, and government) (within 0.8 percentage point of universe), and by urban/rural status (within 0.1 percentage point of universe). Of the providers that were excluded from our final sample, 86 percent were due to having a cost reporting period less than 10 months or greater than 14 months, 10 percent were due to total facility costs or total operating costs not being greater than zero, and 4 percent were due to Medicare general inpatient routine service costs or Medicare PPS payments not being greater than zero.
Additionally, for all of the major cost weights, except Home Office/Related Organization Contract Labor costs, the data are trimmed to remove outliers (a standard statistical process) by: (1) requiring that major expenses (such as Wages and Salaries costs) and total Medicare-allowable costs are greater than zero; and (2) excluding the top and bottom 5 percent of the major cost weight (for example, Wages and Salaries costs as a percent of total Medicare-allowable costs). We noted in the proposed rule that missing values are assumed to be zero, consistent with the methodology for how missing values are treated in the 2018-based SNF market basket methodology.
For the Home Office/Related Organization Contract Labor cost weight, we proposed to first exclude providers whose Home Office/Related Organization Contract Labor costs are greater than Medicare-allowable total costs and then apply a trim that excludes those reporters with a Home Office/Related Organization Contract Labor cost weight above the 99th percentile. This allows providers with no Home Office/Related Organization Contract Labor costs to be included in the Home Office/Related Organization Contract Labor cost weight calculation. If we were to trim the top and bottom Home Office/Related Organization Contract Labor cost weight, we would exclude providers with a cost weight of zero (84 percent of the sample) and the Medicare cost report data (Worksheet S-2 line 45) indicate that not all SNF providers have a home office. Providers without a home office would report administrative costs that might typically be associated with a home office in the Wages and Salaries and Employee Benefits cost weights, or in the residual “All-Other” cost weight if they purchased these types of services from external contractors. We believe the trimming methodology that excludes those who report Home Office/Related Organization Contract Labor costs above the 99th percentile is appropriate as it removes extreme outliers while also allowing providers with zero Home Office/Related Organization Contract Labor costs, which is the majority of providers, to be included in the Home Office/Related Organization Contract Labor cost weight calculation.
The trimming process is done individually for each cost category so that providers excluded from one cost weight calculation are not automatically excluded from another cost weight calculation. We noted in the proposed rule that these trimming methods are the same types of edits performed for the 2018-based SNF market basket, as well as other PPS market baskets (including but not limited to the IPPS market basket and home health market basket). We believe this trimming process improves the accuracy of the data used to compute the major cost weights by removing possible data misreporting.
The final weights of the proposed 2022-based SNF market basket are based on weighted means. For example, the aggregate Wages and Salaries cost weight, after trimming, is equal to the sum of total Medicare-allowable wages and salaries (as defined in the “Wages and Salaries” section that follows) of all providers divided by the sum of total Medicare-allowable costs (as defined in the next paragraph) for all providers in the sample (as defined above in this section). This methodology is consistent with the methodology used to calculate the 2018-based SNF market basket cost weights and other PPS market basket cost weights. We noted in the proposed rule that for each of the cost weights, we evaluated the distribution of providers and costs by region, by ownership-type, and by urban/rural status. For all of the cost weights, the trimmed sample was nationally representative.
For all of the cost weights, we used Medicare-allowable total costs as the denominator (for example, Wages and Salaries cost weight = Wages and Salaries costs divided by Medicare-allowable total costs). Medicare-allowable total costs were equal to total costs (after overhead allocation) from Worksheet B part I, column 18, for lines 30, 40 through 49, 51, 52, and 71 plus estimated Medicaid drug costs, as defined below. We included estimated Medicaid drug costs in the pharmacy cost weight, as well as the denominator for total Medicare-allowable costs. This is the same methodology used for the 2018-based SNF market basket. The inclusion of Medicaid drug costs was finalized in the FY 2008 SNF PPS final rule (72 FR 43425 through 43430), and for the same reasons set forth in that final rule, we proposed to continue to use this methodology in the proposed 2022-based SNF market basket.
We describe the detailed methodology for obtaining costs for each of the eight cost categories determined from the Medicare Cost Report below. The methodology used in the 2018-based SNF market basket can be found in the FY 2022 SNF PPS final rule (86 FR 42446 through 42452).
(1) Wages and Salaries
To derive Wages and Salaries costs for the Medicare-allowable cost centers, we proposed first to calculate total facility wages and salaries costs as reported on Worksheet S-3, part II, column 3, line 1. We then proposed to remove the wages and salaries attributable to non-Medicare-allowable cost centers (that is, excluded areas), as well as a portion of overhead wages and salaries attributable to these excluded areas. Excluded area wages and salaries are equal to wages and salaries as reported on Worksheet S-3, part II, column 3, lines 3, 4, and 7 through 11 plus nursing facility and non-reimbursable salaries from Worksheet A, column 1, lines 31, 32, 50, and 60 through 63.
Overhead wages and salaries are attributable to the entire SNF facility; therefore, we proposed to include only the proportion attributable to the Medicare-allowable cost centers. We proposed to estimate the proportion of overhead wages and salaries attributable to the non-Medicare-allowable costs centers in two steps. First, we proposed to estimate the ratio of excluded area wages and salaries (as defined above) to non-overhead total facility wages and salaries (total facility wages and salaries (Worksheet S-3, part II, column 3, line 1) less total overhead wages and salaries (Worksheet S-3, Part III, column 3, line 14)). Next, we proposed to multiply total overhead wages and salaries by the ratio computed in step 1. We excluded providers whose excluded areas wages and salaries were greater than total facility wages and salaries and/or their excluded area overhead wages and salaries were greater than total facility wages and salaries (about 50 providers). This is the same methodology used to derive Wages and Salaries costs in the 2018-based SNF market basket.
(2) Employee Benefits
Medicare-allowable employee benefits are equal to total facility benefits as reported on Worksheet S-3, part II, column 3, lines 17 through 19 minus non-Medicare-allowable (that is, excluded area) employee benefits and minus a portion of overhead benefits attributable to these excluded areas. Excluded area employee benefits are derived by multiplying total excluded area wages and salaries (as defined above in the `Wages and Salaries' section) times the ratio of total facility benefits to total facility wages and salaries. This ratio of benefits to wages and salaries is defined as total facility benefit costs to total facility wages and salary costs (as reported on Worksheet S-3, part II, column 3, line 1). Likewise, the portion of overhead benefits attributable to the excluded areas is derived by multiplying overhead wages and salaries attributable to the excluded areas (as defined in the “Wages and Salaries” section) times the ratio of total facility benefit costs to total facility wages and salary costs (as defined above). Similar to the Wages and Salaries costs, we excluded providers whose excluded areas benefits were greater than total facility benefits and/or their excluded area overhead benefits were greater than total facility benefits (zero providers were excluded because of this edit). This is the same methodology used to derive Employee Benefits costs in the 2018-based SNF market basket.
(3) Contract Labor
We proposed to derive Medicare-allowable contract labor costs from Worksheet S-3, part II, column 3, line 14, which reflects costs for contracted direct patient care services (that is, nursing, therapeutic, rehabilitative, or diagnostic services furnished under contract rather than by employees and management contract services). This is the same methodology used to derive the Contract Labor costs in the 2018-based SNF market basket.
(4) Pharmaceuticals
We proposed to calculate pharmaceuticals costs using the non-salary costs from the Pharmacy cost center (Worksheet B, part I, column 0, line 11 less Worksheet A, column 1, line 11) and the Drugs Charged to Patients' cost center (Worksheet B, part I, column 0, line 49 less Worksheet A, column 1, line 49). Since these drug costs were attributable to the entire SNF and not limited to Medicare-allowable services, we proposed to adjust the drug costs by the ratio of Medicare-allowable pharmacy total costs (Worksheet B, part I, column 11, for lines 30, 40 through 49, 51, 52, and 71) to total pharmacy costs from Worksheet B, part I, column 11, line 11. Worksheet B, part I allocates the general service cost centers, which are often referred to as “overhead costs” (in which pharmacy costs are included) to the Medicare-allowable and non-Medicare-allowable cost centers. This adjustment was made for those providers who reported Pharmacy cost center expenses. Otherwise, we assumed the non-salary Drugs Charged to Patients costs were Medicare-allowable. Since drug costs for Medicare patients are included in the SNF PPS per diem rate, a provider with Medicare days should have also reported costs in the Drugs Charged to Patient cost center. We found a small number of providers (roughly 90) did not report Drugs Charged to Patients' costs despite reporting Medicare days (an average of about 2,000 Medicare days per provider), and therefore, these providers were excluded from the Pharmaceuticals cost weight calculations. This is the same methodology used for the 2018-based SNF market basket.
Second, as was done for the 2018-based SNF market basket, we proposed to continue to adjust the drug expenses reported on the Medicare cost report to include an estimate of total Medicaid drug costs, which are not represented in the Medicare-allowable drug cost weight. As stated previously in this section, the proposed 2022-based SNF market basket reflects total Medicare-allowable costs (that is, total costs for all payers for those services reimbursable under the SNF PPS). For the FY 2006-based SNF market basket (72 FR 43426), commenters noted that the total pharmaceutical costs reported on the Medicare cost report did not include pharmaceutical costs for dual-eligible Medicaid patients as these were directly reimbursed by Medicaid. Since all of the other cost category weights reflect expenses associated with treating Medicaid patients (including the compensation costs for dispensing these drugs), we made an adjustment to include these Medicaid drug expenses so the market basket cost weights would be calculated consistently.
Similar to the 2018-based SNF market basket, we proposed to estimate Medicaid drug costs based on data representing dual-eligible Medicaid beneficiaries. Medicaid drug costs are estimated by multiplying Medicaid dual-eligible drug costs per day times the number of Medicaid days as reported in the Medicare-allowable skilled nursing cost center (Worksheet S-3, part I, column 5, line 1) in the SNF Medicare cost report. Medicaid dual-eligible drug costs per day (where the day represents an unduplicated drug supply day) were estimated using 2022 Part D claims for those dual-eligible beneficiaries who had a Medicare SNF stay during the year. The total drug costs per unduplicated day for 2022 of $27.43 represented all drug costs (including the drug ingredient cost, the dispensing fee, vaccine administration fee and sales tax) incurred during the 2022 calendar year (CY) for those dual-eligible beneficiaries who had a SNF Medicare stay during CY 2022. Therefore, they include drug costs incurred during a Medicaid SNF stay occurring in CY 2022. By comparison, the 2018-based SNF market basket also relied on data from the Part D claims, which yielded a dual-eligible Medicaid drug cost per day of $24.48 for 2018.
We continue to believe that Medicaid dual-eligible beneficiaries are a reasonable proxy for the estimated drug costs per day incurred by Medicaid patients staying in a skilled nursing unit under a Medicaid stay. The skilled nursing unit is the Medicare-allowable unit in a SNF, which encompasses more skilled nursing and rehabilitative care compared to a nursing facility or long-term care unit. We believe that Medicaid patients receiving this skilled nursing care would on average have similar drug costs per day to dual-eligible Medicare beneficiaries who have received Medicare skilled nursing care in the skilled nursing care unit during the year. We noted in the proposed rule that our previous analysis of the Part D claims data showed that Medicare beneficiaries with a SNF stay during the year have higher drug costs than Medicare patients without a SNF stay during the year. Also, in 2022, dual-eligible beneficiaries with a SNF stay during the year had drug costs per day of $27.43, which were approximately two times higher than the drug costs per day of $15.83 for nondual-eligible beneficiaries with a SNF Part A stay during the year.
The Pharmaceuticals cost weight using only 2022 Medicare cost report data (without the inclusion of the Medicaid dual-eligible drug costs) is 2.0 percent, compared to the proposed Pharmaceuticals cost weight (including the adjustment for Medicaid dual-eligible drug costs) of 6.4 percent. The 2018-based SNF market basket had a Pharmaceuticals cost weight using only 2018 Medicare cost report data without the inclusion of the Medicaid dual-eligible drug costs of 2.6 percent and a total Pharmaceuticals cost weight of 7.5 percent. Therefore, the 1.1 percentage point decrease in the Pharmaceuticals cost weight between 2018 and 2022 is a result of a 0.5-percentage point decrease in the Medicaid dual-eligible drug cost weight (reflecting the 12 percent increase in the Medicaid dual-eligible drug costs per day, and a 14 percent decrease in Medicaid inpatient days between 2018 and 2022) and a 0.6-percentage point decrease in the Medicare cost report drug cost weight. The decrease in the Medicare cost report drug cost weight was consistent, in aggregate, across urban and rural status SNFs, as well as across for-profit, government, and nonprofit ownership type SNFs.
(5) Professional Liability Insurance
We proposed to calculate the professional liability insurance (PLI) costs from Worksheet S-2 of the Medicare cost reports as the sum of premiums; paid losses; and self-insurance (Worksheet S-2, Part I, columns 1 through 3, line 41). This was the same methodology used to derive the Professional Liability costs for the 2018-based SNF market basket.
About 60 percent of SNFs (about 7,700) reported professional liability costs. After trimming, about 6,900 (reflecting about 730,000 Skilled Nursing unit beds) were included in the calculation of the PLI cost weight for the proposed 2022-based SNF market basket. These providers treated roughly 750,000 Medicare beneficiaries and had a Medicare length of stay (LOS) of 58 days, a skilled nursing unit occupancy rate of 72 percent, and an average skilled nursing unit bed size of 106 beds, which are all consistent with the national averages. We also verified that this sample of providers are representative of the national distribution of providers by ownership-type, urban/rural status, and region.
We believe the Medicare cost report data continues to be the most appropriate data source to calculate the PLI cost weight for the proposed 2022-based SNF market basket as it is representative of SNFs serving Medicare beneficiaries and reflects PLI costs (premiums, paid losses, and self-insurance) incurred during the provider's cost reporting year. A fuller discussion of the Medicare cost report data on PLI costs compared to other sources is available in the FY 2022 SNF PPS final rule (86 FR 42448).
(6) Capital-Related
We proposed to derive the Medicare-allowable capital-related costs from Worksheet B, part II, column 18 for lines 30, 40 through 49, 51, 52, and 71. This is the same methodology to derive capital-related costs used in the 2018-based SNF market basket.
(7) Home Office/Related Organization Contract Labor Costs
We proposed to calculate Medicare-allowable Home Office/Related Organization Contract Labor costs to be equal to data reported on Worksheet S-3, part II, column 3, line 16. About 7,100 providers (about 54 percent) in 2022 reported having a home office (as reported on Worksheet S-2, part I, line 45) about the same share of providers as those in the 2018-based SNF market basket. As outlined in section V.A.1. of the proposed rule, providers without a home office can incur these expenses directly by having their own staff, for which the costs would be included in the Wages and Salaries and Employee Benefits cost weights. Alternatively, providers without a home office could also purchase related services from external contractors for which these expenses would be captured in the residual “All-Other” cost weight. For this reason, unlike the other major cost weights described previously, we did not exclude providers that did not report Home Office/Related Organization Contract Labor costs. This is the same methodology that was used in the 2018-based SNF market basket.
(8) All Other (Residual)
The “All Other” cost weight is a residual, calculated by subtracting the major cost weights (Wages and Salaries, Employee Benefits, Contract Labor, Pharmaceuticals, Professional Liability Insurance, Capital-Related, and Home Office/Related Organization Contract Labor) from 100.
We did not receive public comments on our proposed major cost weights, nor their respective methodologies of derivation. For the reasons discussed above and in the FY 2025 SNF PPS proposed rule, we are finalizing the major cost weights as proposed, without modification.
Table 11 shows the major cost categories and their respective cost weights as derived from the 2022 Medicare cost reports.
As we did for the 2018-based SNF market basket (86 FR 42449), we proposed to allocate contract labor costs to the Wages and Salaries and Employee Benefits cost weights based on their relative proportions under the assumption that contract labor costs are composed of both wages and salaries and employee benefits. The contract labor allocation proportion for wages and salaries is equal to the Wages and Salaries cost weight as a percent of the sum of the Wages and Salaries cost weight and the Employee Benefits cost weight. Using the 2022 Medicare cost report data, this percentage is 85 percent (1 percentage point higher than the percentage in the 2018-based SNF market basket); therefore, we proposed to allocate approximately 85 percent of the Contract Labor cost weight to the Wages and Salaries cost weight and 15 percent to the Employee Benefits cost weight.
We did not receive public comments on our proposed allocation of contract labor costs to Wages and Salaries and Employee Benefits. For the reasons discussed above and in the FY 2025 SNF PPS proposed rule, we are finalizing the allocation methodology and percentages as proposed, without modification.
Table 12 shows the Wages and Salaries and Employee Benefits cost weights after contract labor allocation for the 2022-based SNF market basket and the 2018-based SNF market basket.
Compared to the 2018-based SNF market basket, the Wages and Salaries cost weight and the Employee Benefits cost weight as calculated directly from the Medicare cost reports each decreased by 0.8 percentage point. The Contract Labor cost weight increased 2.6 percentage points and so in aggregate, the Compensation cost weight increased 1.0 percentage point from 60.2 percent to 61.2 percent.
b. Derivation of the Detailed Operating Cost Weights
To further divide the “All Other” residual cost weight estimated from the 2022 Medicare cost report data into more detailed cost categories, we proposed to use the 2017 Benchmark I-O “The Use Table (Supply-Use Framework)” for Nursing and Community Care Facilities industry (NAICS 623A00), published by the Census Bureau's, Bureau of Economic Analysis (BEA). These data are publicly available at https://www.bea.gov/industry/input-output-accounts-data. The BEA Benchmark I-O data are generally scheduled for publication every 5 years with 2017 being the most recent year for which data are available. The 2017 Benchmark I-O data are derived from the 2017 Economic Census and are the building blocks for BEA's economic accounts; therefore, they represent the most comprehensive and complete set of data on the economic processes or mechanisms by which output is produced and distributed.[1] BEA also produces Annual I-O estimates. However, while based on a similar methodology, these estimates are less comprehensive and provide less detail than benchmark data. Additionally, the annual I-O data are subject to revision once benchmark data become available. For these reasons, we proposed to inflate the 2017 Benchmark I-O data aged forward to 2022 by applying the annual price changes from the respective price proxies to the appropriate market basket cost categories that are obtained from the 2017 Benchmark I-O data. Next, the relative shares of the cost shares that each cost category represents to the total residual I-O costs are calculated. These resulting 2022 cost shares of the I-O data are applied to the “All Other” residual cost weight to obtain detailed cost weights for the residual costs for the proposed 2022-based SNF market basket. For example, the cost for Food: Direct Purchases represents 12.8 percent of the sum of the “All Other” 2017 Benchmark I-O Expenditures inflated to 2022. Therefore, the Food: Direct Purchases cost weight is 2.8 percent of the proposed 2022-based SNF market basket (12.8 percent × 22.2 percent = 2.8 percent). For the 2018-based SNF market basket (86 FR 42449), we used a similar methodology utilizing the 2012 Benchmark I-O data (aged to 2018).
Using this methodology, we proposed to derive 19 detailed SNF market basket cost category weights from the proposed 2022-based SNF market basket “All Other” residual cost weight (22.2 percent). These categories are: (1) Fuel: Oil and Gas; (2) Electricity and Other Non-Fuel Utilities; (3) Food: Direct Purchases; (4) Food: Contract Services; (5) Chemicals; (6) Medical Instruments and Supplies; (7) Rubber and Plastics; (8) Paper and Printing Products; (9) Apparel; (10) Machinery and Equipment; (11) Miscellaneous Products; (12) Professional Fees: Labor-Related; (13) Administrative and Facilities Support Services; (14) Installation, Maintenance, and Repair Services; (15) All Other: Labor-Related Services; (16) Professional Fees: Nonlabor-Related; (17) Financial Services; (18) Telephone Services; and (19) All Other: Nonlabor-Related Services. These are the same detailed cost categories as those that were used in the 2018-based SNF market basket.
We noted in the proposed rule that the machinery and equipment expenses are for equipment that is paid for in a given year and not depreciated over the asset's useful life. Depreciation expenses for movable equipment are accounted for in the capital component of the proposed 2022-based SNF market basket (described in section V.A.1.c. of the proposed rule).
We did not receive any public comments on our proposed methodology for deriving the detailed operating cost weights. Therefore, for the reasons discussed above and in the FY 2025 SNF PPS proposed rule, we are finalizing the detailed operating cost weights and methodology as proposed, without modification.
c. Derivation of the Detailed Capital Cost Weights
Similar to the 2018-based SNF market basket, we further divided the Capital-related cost weight into: Depreciation, Interest, Lease and Other Capital-related cost weights.
We calculated the depreciation cost weight (that is, depreciation costs excluding leasing costs) using depreciation costs from Worksheet S-2, column 1, lines 20 and 21. Since the depreciation costs reflect the entire SNF facility (Medicare and non-Medicare-allowable units), we used total facility capital costs (Worksheet B, Part I, column 18, line 100) as the denominator. This methodology assumes that the depreciation of an asset is the same regardless of whether the asset was used for Medicare or non-Medicare patients. This methodology yielded depreciation costs as a percent of capital costs of 22.6 percent for 2022. We then apply this percentage to the proposed 2022-based SNF market basket Medicare-allowable Capital-related cost weight of 8.3 percent, yielding a proposed Medicare-allowable depreciation cost weight (excluding leasing expenses, which is described in more detail below) of 1.9 percent for 2022. To further disaggregate the Medicare-allowable depreciation cost weight into fixed and movable depreciation, we proposed to use the 2022 SNF Medicare cost report data for end-of-the-year capital asset balances as reported on Worksheet A-7. The 2022 SNF Medicare cost report data showed a fixed/movable split of 86/14. The 2018-based SNF market basket, which utilized the same data from the 2018 Medicare cost reports, also had a fixed/movable split of 86/14.
We derived the interest expense share of capital-related expenses from 2022 SNF Medicare cost report data, specifically from Worksheet A, column 2, line 81. Similar to the depreciation cost weight, we calculated the interest cost weight using total facility capital costs. This methodology yielded interest costs as a percent of capital costs of 17.7 percent for 2022. We then apply this percentage to the proposed 2022-based SNF market basket Medicare-allowable Capital-related cost weight of 8.3 percent, yielding a Medicare-allowable interest cost weight (excluding leasing expenses) of 1.5 percent. As done with the last rebasing (86 FR 42450), we proposed to determine the split of interest expense between for-profit and not-for-profit facilities based on the distribution of long-term debt outstanding by type of SNF (for-profit or not-for-profit/government) from the 2022 SNF Medicare cost report data. We estimated the split between for-profit and not-for-profit interest expense to be 30/70 percent compared to the 2018-based SNF market basket with 25/75 percent.
Because the detailed data were not available in the Medicare cost reports, we used the most recent 2021 Census Bureau Service Annual Survey (SAS) data to derive the capital-related expenses attributable to leasing and other capital-related expenses. The 2018-based SNF market basket used the 2017 SAS data.
Based on the 2021 SAS data, we determined that leasing expenses are 65 percent of total leasing and capital-related expenses costs. In the 2018-based SNF market basket, leasing costs represent 62 percent of total leasing and capital-related expenses costs. We then apply this percentage to the 2022-based SNF market basket residual Medicare-allowable capital costs of 4.9 percent derived from subtracting the Medicare-allowable depreciation cost weight and Medicare-allowable interest cost weight from the 2022-based SNF market basket of total Medicare-allowable capital cost weight (8.3 percent−1.9 percent−1.5 percent = 4.9 percent). This produces the 2022-based SNF Medicare-allowable leasing cost weight of 3.2 percent and all-other capital-related cost weight of 1.7 percent.
Lease expenses are not broken out as a separate cost category in the SNF market basket, but are distributed among the cost categories of depreciation, interest, and other capital-related expenses, reflecting the assumption that the underlying cost structure and price movement of leasing expenses is similar to capital costs in general. As was done with past SNF market baskets and other PPS market baskets, we assumed 10 percent of lease expenses are overhead and assigned them to the other capital-related expenses cost category. This is based on the assumption that leasing expenses include not only depreciation, interest, and other capital-related costs but also additional costs paid to the lessor. We distributed the remaining lease expenses to the three cost categories based on the proportion of depreciation, interest, and other capital-related expenses to total capital costs, excluding lease expenses.
We did not receive any public comments on our proposed methodology for deriving the detailed capital cost weights. Therefore, for the reasons discussed above and in the FY 2025 SNF PPS proposed rule, we are finalizing the detailed capital cost weights and methodology as proposed, without modification.
Table 13 shows the capital-related expense distribution (including expenses from leases) in the 2022-based SNF market basket and the 2018-based SNF market basket.
Table 14 presents the 2022-based SNF market basket and the 2018-based SNF market basket cost categories and cost weights.
2. Price Proxies Used To Measure Operating Cost Category Growth
After developing the 27 cost weights for the 2022-based SNF market basket, we selected the most appropriate wage and price proxies currently available to represent the rate of change for each cost category. With four exceptions (three for the capital-related expenses cost categories and one for PLI), we base the wage and price proxies on Bureau of Labor Statistics (BLS) data, and group them into one of the following BLS categories:
- Employment Cost Indexes. Employment Cost Indexes (ECIs) measure the rate of change in employment wage rates and employer costs for employee benefits per hour worked. These indexes are fixed-weight indexes and strictly measure the change in wage rates and employee benefits per hour. ECIs are superior to Average Hourly Earnings (AHE) as price proxies for input price indexes because they are not affected by shifts in occupation or industry mix, and because they measure pure price change and are available by both occupational group and by industry. The industry ECIs are based on the NAICS and the occupational ECIs are based on the Standard Occupational Classification System (SOC).
- Producer Price Indexes. Producer Price Indexes (PPIs) measure the average change over time in the selling prices received by domestic producers for their output. The prices included in the PPI are from the first commercial transaction for many products and some services ( https://www.bls.gov/ppi/).
- Consumer Price Indexes. Consumer Price Indexes (CPIs) measure the average change over time in the prices paid by urban consumers for a market basket of consumer goods and services ( https://www.bls.gov/cpi/). CPIs are only used when the purchases are similar to those of retail consumers rather than purchases at the producer level, or if no appropriate PPIs are available.
We evaluate the price proxies using the criteria of reliability, timeliness, availability, and relevance:
- Reliability. Reliability indicates that the index is based on valid statistical methods and has low sampling variability. Widely accepted statistical methods ensure that the data were collected and aggregated in a way that can be replicated. Low sampling variability is desirable because it indicates that the sample reflects the typical members of the population. (Sampling variability is variation that occurs by chance because only a sample was surveyed rather than the entire population.)
- Timeliness. Timeliness implies that the proxy is published regularly, preferably at least once a quarter. The market baskets are updated quarterly, and therefore, it is important for the underlying price proxies to be up-to-date, reflecting the most recent data available. We believe that using proxies that are published regularly (at least quarterly, whenever possible) helps to ensure that we are using the most recent data available to update the market basket. We strive to use publications that are disseminated frequently, because we believe that this is an optimal way to stay abreast of the most current data available.
- Availability. Availability means that the proxy is publicly available. We prefer that our proxies are publicly available because this will help ensure that our market basket updates are as transparent to the public as possible. In addition, this enables the public to be able to obtain the price proxy data on a regular basis.
- Relevance. Relevance means that the proxy is applicable and representative of the cost category weight to which it is applied.
We believe that the CPIs, PPIs, and ECIs that we have selected meet these criteria. Therefore, we believe that they continue to be the best measure of price changes for the cost categories to which they would be applied.
Table 19 lists all price proxies for the 2022-based SNF market basket. Below is a detailed explanation of the price proxies we proposed to use for each operating cost category.
a. Wages and Salaries
We proposed to use the ECI for Wages and Salaries for Private Industry Workers in Nursing Care Facilities (NAICS 6231; BLS series code CIU2026231000000I) to measure price growth of this category. NAICS 623 includes facilities that provide a mix of health and social services, with many of the health services requiring some level of nursing services. Within NAICS 623 is NAICS 6231, which includes nursing care facilities primarily engaged in providing inpatient nursing and rehabilitative services. These facilities, which are most comparable to Medicare-certified SNFs, provide skilled nursing and continuous personal care services for an extended period of time, and, therefore, have a permanent core staff of registered or licensed practical nurses. This is the same index used in the 2018-based SNF market basket.
b. Employee Benefits
We proposed to use the ECI for Benefits for Nursing Care Facilities (NAICS 6231) to measure price growth of this category. The ECI for Benefits for Nursing Care Facilities is calculated using BLS's total compensation (BLS series ID CIU2016231000000I) for nursing care facilities series and the relative importance of wages and salaries within total compensation. We believe this constructed ECI series is technically appropriate for the reason stated previously in the Wages and Salaries price proxy section of this final rule. This is the same index used in the 2018-based SNF market basket.
c. Electricity and Other Non-Fuel Utilities
We proposed to use the PPI Commodity for Commercial Electric Power (BLS series code WPU0542) to measure the price growth of this cost category as Electricity costs account for 93 percent of these expenses. This is the same index used for the Electricity cost category in the 2018-based SNF market basket.
d. Fuel: Oil and Gas
We proposed to use a blended proxy composed of the PPI Industry for Petroleum Refineries (NAICS 324110) (BLS series code PCU32411-32411), the PPI Commodity for Natural Gas (NAICS 221200)(BLS series code WPU0531), and the PPI for Other Petroleum and Coal Products manufacturing (NAICS 324190)(BLS series code PCU32419-32419).
Our analysis of 2017 Benchmark I-O data for Nursing and Community Care Facilities found that these three NAICS industries account for approximately 93 percent of SNF Fuel: Oil and Gas expenses. The remaining 7 percent of SNF Fuel: Oil and Gas expenses are for two other incidental NAICS industries including Coal Mining and Petrochemical Manufacturing. We proposed to create a blended index based on the three NAICS Fuel: Oil and Gas expenses listed above that account for 93 percent of SNF Fuel: Oil and Gas expenses. We created this blend based on each NAICS' expenses as a share of their sum. These expenses as a share of their sum are listed in Table 15.
The 2018-based SNF market basket used a blended Fuel: Oil and Gas proxy that was based on 2012 Benchmark I-O data. We believe the Fuel: Oil and Gas blended index for the 2022-based SNF market basket is technically appropriate as it reflects more recent data on SNFs purchasing patterns. Table 15 provides the weights for the 2022- and 2018-based blended Fuel: Oil and Gas index.
e. Professional Liability Insurance
We proposed to use the CMS Hospital Professional Liability Insurance Index to measure price growth of this category. We were unable to find a reliable data source that collects SNF-specific PLI data. Therefore, we proposed to use the CMS Hospital Professional Liability Index, which tracks price changes for commercial insurance premiums for a fixed level of coverage, holding non-price factors constant (such as a change in the level of coverage). This is the same index used in the 2018-based SNF market basket. We believe this is an appropriate proxy to measure the price growth associated of SNF PLI as it captures the price inflation associated with other medical institutions that serve Medicare patients.
Comment: One commenter mentioned a 2006 case study on the nursing home liability insurance market in Florida that relied on information from the National Conference of State Legislatures Health Policy Tracking Service and suggested that CMS should be looking for credible sources of information about SNF liability insurance rather than using the CMS Hospital Professional Liability Insurance Index as this market basket's price proxy.
Response: The criteria we use to evaluate and select price proxies are: timeliness (published and available on a regular basis, preferably at least quarterly, with little lag), reliability (consistent historical time-series as well as being technically and methodologically sound), availability (the proxy is publicly available), and relevance (the proxy is applicable and representative of the cost category weight to which it is applied). While we are unaware of any data sources that would meet these criteria and serve as an appropriate substitute at this time, we are interested in information on this topic and will continue to search for, and remain open to, any credible data source that meets the aforementioned criteria. Nonetheless, we continue to believe that the CMS Hospital Professional Liability Insurance Index is an appropriate price proxy as it captures the price inflation associated with other medical institutions that serve Medicare patients, which includes hospital-based SNFs. Any changes to this price proxy in the future would be set forth through notice and comment rulemaking.
f. Pharmaceuticals
We proposed to use the PPI Commodity for Pharmaceuticals for Human Use, Prescription (BLS series code WPUSI07003) to measure the price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
g. Food: Direct Purchases
We proposed to use the PPI Commodity for Processed Foods and Feeds (BLS series code WPU02) to measure the price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
h. Food: Contract Services
We proposed to use the CPI All Urban for Food Away From Home (All Urban Consumers) (BLS series code CUUR0000SEFV) to measure the price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
i. Chemicals
For measuring price change in the Chemicals cost category, we proposed to use a blended PPI composed of the Industry PPIs for Other Basic Organic Chemical Manufacturing (NAICS 325190) (BLS series code PCU32519-32519), Soap and Cleaning Compound Manufacturing (NAICS 325610) (BLS series code PCU32561-32561), and All Other Chemical Product and Preparation Manufacturing (NAICS 3259A0) (BLS series code PCU325998325998).
Using the 2017 Benchmark I-O data, we found that these three NAICS industries accounted for approximately 95 percent of SNF chemical expenses. The remaining 5 percent of SNF chemical expenses are for three other incidental NAICS chemicals industries such as Paint and Coating Manufacturing. We proposed to create a blended index based on the three NAICS chemical expenses listed above that account for 95 percent of SNF chemical expenses. We create this blend based on each NAICS' expenses as a share of their sum. These expenses as a share of their sum are listed in Table 16.
The 2018-based SNF market basket used a blended chemical proxy that was based on 2012 Benchmark I-O data. We believe the chemical blended index for the 2022-based SNF market basket is technically appropriate as it reflects more recent data on SNFs purchasing patterns. Table B6 provides the weights for the 2022-based blended chemical index and the 2018-based blended chemical index.
j. Medical Instruments and Supplies
For measuring price change in the Medical Instruments and Supplies cost category, we proposed to use a blended proxy. The 2017 Benchmark I-O data shows 62 percent of medical instruments and supply costs are for Surgical and medical instrument manufacturing costs (NAICS 339112) and 38 percent are for Surgical appliance and supplies manufacturing costs (NAICS 339113). To proxy the price changes associated with NAICS 339112, we proposed using the PPI—Commodity—Surgical and medical instruments (BLS series code WPU1562). To proxy the price changes associated with NAICS 339113, we proposed to use 50 percent for the PPI—Commodity—Medical and surgical appliances and supplies (BLS series code WPU1563) and 50 percent for the PPI Commodity data for Miscellaneous products—Personal safety equipment and clothing (BLS series code WPU1571). The latter price proxy would reflect personal protective equipment including but not limited to face shields and protective clothing. The 2017 Benchmark I-O data does not provide specific expenses for personal protective equipment (which would be reflected in the NAICS 339113 expenses); however, we recognize that this category reflects costs faced by SNFs. In absence of any specific cost data on personal protective equipment, we proposed to include the PPI Commodity data for Miscellaneous products—Personal safety equipment and clothing (BLS series code WPU1571) in the blended proxy for Medical Instruments and Supplies cost category with a weight of 19 percent (that is, 50 percent of the NAICS 339113 expenses as a percent of the sum of NAICS 339113 and NAICS 339112 expenses from the I-O).
The 2018-based SNF market basket used a blended Medical Instruments and Supplies proxy that was based on 2012 Benchmark I-O data. We believe the blended index for the 2022-based SNF market basket is technically appropriate as it reflects more recent data on SNFs purchasing patterns. Table 17 provides the Medical Instruments and Supplies cost weight blended price proxy.
k. Rubber and Plastics
We proposed to use the PPI Commodity for Rubber and Plastic Products (BLS series code WPU07) to measure price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
l. Paper and Printing Products
We proposed to use a 86/14 blend of the PPI Commodity for Converted Paper and Paperboard Products (BLS series code WPU0915) and the PPI Commodity for Publications Printed Matter and Printing Material (BLS Series Code WPU094) to measure the price growth of this cost category. The 2017 Benchmark I-O data shows that 86 percent of paper and printing expenses are for paper manufacturing (NAICS 322) and the remaining expenses are for Printing (NAICS 323110). The 2018-based SNF market basket used the PPI Commodity for Converted Paper and Paperboard Products (BLS series code WPU0915) to measure the price growth of this cost category.
m. Apparel
We proposed to use the PPI Commodity for Apparel (BLS series code WPU0381) to measure the price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
n. Machinery and Equipment
We proposed to use the PPI Commodity for Machinery and Equipment (BLS series code WPU11) to measure the price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
o. Miscellaneous Products
For measuring price change in the Miscellaneous Products cost category, we proposed to use the PPI Commodity for Finished Goods less Food and Energy (BLS series code WPUFD4131). Both food and energy are already adequately represented in separate cost categories and should not also be reflected in this cost category. This is the same index used in the 2018-based SNF market basket.
p. Professional Fees: Labor-Related
We proposed to use the ECI for Total Compensation for Private Industry Workers in Professional and Related (BLS series code CIU2010000120000I) to measure the price growth of this category. This is the same index used in the 2018-based SNF market basket.
q. Administrative and Facilities Support Services
We proposed to use the ECI for Total Compensation for Private Industry Workers in Office and Administrative Support (BLS series code CIU2010000220000I) to measure the price growth of this category. This is the same index used in the 2018-based SNF market basket.
r. Installation, Maintenance and Repair Services
We proposed to use the ECI for Total Compensation for All Civilian Workers in Installation, Maintenance, and Repair (BLS series code CIU1010000430000I) to measure the price growth of this new cost category. This is the same index used in the 2018-based SNF market basket.
s. All Other: Labor-Related Services
We proposed to use the ECI for Total Compensation for Private Industry Workers in Service Occupations (BLS series code CIU2010000300000I) to measure the price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
t. Professional Fees: Non-Labor-Related
We proposed to use the ECI for Total Compensation for Private Industry Workers in Professional and Related (BLS series code CIU2010000120000I) to measure the price growth of this category. This is the same index used in the 2018-based SNF market basket.
u. Financial Services
We proposed to use the ECI for Total Compensation for Private Industry Workers in Financial Activities (BLS series code CIU201520A000000I) to measure the price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
v. Telephone Services
We proposed to use the CPI All Urban for Telephone Services (BLS series code CUUR0000SEED) to measure the price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
w. All Other: Non-Labor-Related Services
We proposed to use the CPI All Urban for All Items Less Food and Energy (BLS series code CUUR0000SA0L1E) to measure the price growth of this cost category. This is the same index used in the 2018-based SNF market basket.
After consideration of the public comments we received, for the reasons discussed above and in the FY 2025 SNF PPS proposed rule, we are finalizing the price proxies of the operating cost categories as proposed, without modification.
3. Price Proxies Used To Measure Capital Cost Category Growth
We proposed to apply the same capital price proxies as were used in the 2018-based SNF market basket, and below is a detailed explanation of the price proxies used for each capital cost category. We also proposed to continue to vintage weight the capital price proxies for Depreciation and Interest to capture the long-term consumption of capital. This vintage weighting method is the same method that was used for the 2018-based SNF market basket and is described below.
- Depreciation—Building and Fixed Equipment: We proposed to use the BEA Chained Price Index for Private Fixed Investment in Structures, Nonresidential, Hospitals and Special Care (BEA Table 5.4.4. Price Indexes for Private Fixed Investment in Structures by Type). This BEA index is intended to capture prices for construction of facilities such as hospitals, nursing homes, hospices, and rehabilitation centers. This is the same index used in the 2018-based SNF market basket.
- Depreciation—Movable Equipment: We proposed to use the PPI Commodity for Machinery and Equipment (BLS series code WPU11). This price index reflects price inflation associated with a variety of machinery and equipment that would be utilized by SNFs, including but not limited to medical equipment, communication equipment, and computers. This is the same index used in the 2018-based SNF market basket.
- Nonprofit Interest: We proposed to use the average yield on Municipal Bonds (Bond Buyer 20-bond index). This is the same index used in the 2018-based SNF market basket.
- For-Profit Interest: For the For-Profit Interest cost category, we proposed to use the iBoxx AAA Corporate Bond Yield index. This is the same index used in the 2018-based SNF market basket.
- Other Capital: Since this category includes fees for insurances, taxes, and other capital-related costs, we proposed to use the CPI for Rent of Primary Residence (BLS series code CUUS0000SEHA), which would reflect the price growth of these costs. This is the same index used in the 2018-based SNF market basket.
We believe that these price proxies are the most appropriate proxies for SNF capital costs that meet our selection criteria of relevance, timeliness, availability, and reliability.
As stated previously in this final rule, we proposed to continue to vintage weight the capital price proxies for Depreciation and Interest to capture the long-term consumption of capital. To capture the long-term nature, the price proxies are vintage-weighted and the vintage weights are calculated using a two-step process. First, we determine the expected useful life of capital and debt instruments held by SNFs. Second, we identify the proportion of expenditures within a cost category that is attributable to each individual year over the useful life of the relevant capital assets, or the vintage weights.
We rely on Bureau of Economic Analysis (BEA) fixed asset data to derive the useful lives of both fixed and movable capital, which is the same data source used to derive the useful lives for the 2018-based SNF market basket. The specifics of the data sources used are explained below.
a. Calculating Useful Lives for Movable and Fixed Assets
Estimates of useful lives for movable and fixed assets for the 2022-based SNF market basket are 9 and 27 years, respectively. These estimates are based on three data sources from the BEA: (1) current-cost average age; (2) historical-cost average age; and (3) industry-specific current cost net stocks of assets.
BEA current-cost and historical-cost average age data by asset type are not available by industry but are published at the aggregate level for all industries. The BEA does publish current-cost net capital stocks at the detailed asset level for specific industries. There are 64 detailed movable assets (including intellectual property) and there are 32 detailed fixed assets in the BEA estimates. Since we seek aggregate useful life estimates applicable to SNFs, we developed a methodology to approximate movable and fixed asset ages for nursing and residential care services (NAICS 623) using the published BEA data. For the 2022-based SNF market basket, we use the current-cost average age for each asset type from the BEA fixed assets Table 2.9 for all assets and weight them using current-cost net stock levels for each of these asset types in the nursing and residential care services industry, NAICS 6230. For example, nonelectro medical equipment current-cost net stock (accounting for about 29 percent of total movable equipment current-cost net stock in 2022 is multiplied by an average age of 4.8 years for nonelectro medical equipment for all industries. Current-cost net stock levels are available for download from the BEA website at https://apps.bea.gov/iTable/index_FA.cfm. We then aggregate the “weighted” current-cost net stock levels (average age multiplied by current-cost net stock) into movable and fixed assets for NAICS 6230. We then adjust the average ages for movable and fixed assets by the ratio of historical-cost average age (Table 2.10) to current-cost average age (Table 2.9).
This produces historical cost average age data for fixed (structures) and movable (equipment and intellectual property) assets specific to NAICS 6230 of 13.6 and 4.4 years for 2022, respectively. This reflects the average age of an asset at a given point in time, whereas we want to estimate a useful life of the asset. To do this, we multiply each of the average age estimates by two to convert to average useful lives with the assumption that the average age reflects the midpoint of useful life and is normally distributed (about half of the assets are below the average at a given point in time, and half above the average at a given point in time). This produces estimates of likely useful lives of 27.2 and 8.8 years for fixed and movable assets, which we round to 27 and 9 years, respectively. We proposed an interest vintage weight time span of 25 years, obtained by weighting the fixed and movable vintage weights (27 years and 9 years, respectively) by the fixed and movable split (86 percent and 14 percent, respectively). This is the same methodology used for the 2018-based SNF market basket, which had useful lives of 26 years and 9 years for fixed and movable assets, respectively.
b. Constructing Vintage Weights
Given the expected useful life of capital (fixed and movable assets) and debt instruments, we must determine the proportion of capital expenditures attributable to each year of the expected useful life for each of the three asset types: building and fixed equipment, movable equipment, and interest. These proportions represent the vintage weights. We were not able to find a historical time series of capital expenditures by SNFs. Therefore, we approximated the capital expenditure patterns of SNFs over time using alternative SNF data sources. For building and fixed equipment, we used the stock of beds in nursing homes from the National Nursing Home Survey (NNHS) conducted by the National Center for Health Statistics (NCHS) for 1962 through 1999. For 2000 through 2018, we extrapolated the 1999 bed data forward using measurements of the moving average rate of growth in the number of beds as reported in SNF Medicare cost report data on Worksheet S-3, part I, column 1, line 8. A more detailed discussion of this methodology was published in the FY 2022 SNF final rule (86 FR 42457). We proposed to continue this methodology for the 2022-based SNF market basket by extrapolating the 2018 bed data forward using the average growth in the number of beds over the 2019 to 2022 time period. We then proposed to use the change in the stock of beds each year to approximate building and fixed equipment purchases for that year. This procedure assumes that bed growth reflects the growth in capital-related costs in SNFs for building and fixed equipment. We believe that this assumption is reasonable because the number of beds reflects the size of a SNF, and as a SNF adds beds, it also likely adds fixed capital.
As was done for the 2018-based SNF market basket (as well as prior market baskets), we proposed to estimate movable equipment purchases based on the ratio of ancillary costs to routine costs. The time series of the ratio of ancillary costs to routine costs for SNFs measures changes in intensity in SNF services, which are assumed to be associated with movable equipment purchase patterns. The assumption here is that as ancillary costs increase compared to routine costs, the SNF caseload becomes more complex and would require more movable equipment. The lack of movable equipment purchase data for SNFs over time required us to use alternative SNF data sources. A more detailed discussion of this methodology was published in the FY 2008 SNF final rule (72 FR 43428). We believe the resulting two time series, determined from beds and the ratio of ancillary to routine costs, reflect real capital purchases of building and fixed equipment and movable equipment over time.
To obtain nominal purchases, which are used to determine the vintage weights for interest, we converted the two real capital purchase series from 1963 through 2022 determined above to nominal capital purchase series using their respective price proxies (the BEA Chained Price Index for Nonresidential Construction for Hospitals & Special Care Facilities and the PPI for Machinery and Equipment). We then combined the two nominal series into one nominal capital purchase series for 1963 through 2022. Nominal capital purchases are needed for interest vintage weights to capture the value of debt instruments.
Once we created these capital purchase time series for 1963 through 2022, we averaged different periods to obtain an average capital purchase pattern over time: (1) for building and fixed equipment, we averaged 34, 27-year periods; (2) for movable equipment, we averaged 52, 9-year periods; and (3) for interest, we averaged 36, 25-year periods. We calculate the vintage weight for a given year by dividing the capital purchase amount in any given year by the total amount of purchases during the expected useful life of the equipment or debt instrument.
We did not receive any public comments on our proposed price proxies used for each of the detailed capital cost categories or on our methodology for deriving the vintage weights. For the reasons discussed above and in the FY 2025 SNF PPS proposed rule, we are finalizing the price proxies of the capital cost categories, the vintage weights, and the methodology for deriving the vintage weights, as proposed without modification.
The vintage weights for the 2022-based SNF market basket and the 2018-based SNF market basket are presented in Table 18.
The process of creating vintage-weighted price proxies requires applying the vintage weights to the price proxy index where the last applied vintage weight in Table 18 is applied to the most recent data point. We have provided on the CMS website an example of how the vintage weighting price proxies are calculated, using example vintage weights and example price indices. The example can be found at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/MarketBasketResearch.html in the zip file titled “Weight Calculations as described in this IPPS FY 2010 Proposed Rule.”
After consideration of public comments, we are finalizing the 2022-based SNF market basket as proposed. Table 19 shows all the price proxies for the 2022-based SNF market basket.
4. Labor-Related Share
We define the labor-related share (LRS) as those expenses that are labor-intensive and vary with, or are influenced by, the local labor market. Each year, we calculate a revised labor-related share based on the relative importance of labor-related cost categories in the input price index. Effective for FY 2025, we proposed to revise and update the labor-related share to reflect the relative importance of the 2022-based SNF market basket cost categories that we believe are labor-intensive and vary with, or are influenced by, the local labor market. For the 2022-based SNF market basket these are: (1) Wages and Salaries (including allocated contract labor costs as described above); (2) Employee Benefits (including allocated contract labor costs as described above); (3) Professional Fees: Labor-Related; (4) Administrative and Facilities Support Services; (5) Installation, Maintenance, and Repair Services; (6) All Other: Labor-Related Services; and (7) a proportion of capital-related expenses. We proposed to continue to include a proportion of capital-related expenses because a portion of these expenses are deemed to be labor-intensive and vary with, or are influenced by, the local labor market. For example, a proportion of construction costs for a medical building would be attributable to local construction workers' compensation expenses.
Consistent with previous SNF market basket revisions and rebasings, the All Other: Labor-related services cost category is mostly comprised of building maintenance and security services (including, but not limited to, landscaping services, janitorial services, waste management services services) and dry cleaning and laundry services. Because these services tend to be labor-intensive and are mostly performed at the SNF facility or in the local area (and therefore, unlikely to be purchased in the national market), we believe that they meet our definition of labor-related services.
These are the same cost categories we have included in the labor-related share for the 2018-based SNF market basket rebasing (86 FR 42461), as well as the same categories included in the labor-related share for the 2021-based inpatient rehabilitation facility (IRF) market basket (88 FR 50984), and 2021-based inpatient psychiatric facility (IPF) market basket (88 FR 51078).
As discussed in the FY 2022 SNF PPS final rule (86 FR 42462), in an effort to determine more accurately the share of nonmedical professional fees (included in the 2022-based SNF market basket Professional Fees cost categories) that should be included in the labor-related share, we surveyed SNFs regarding the proportion of those fees that are attributable to local firms and the proportion that are purchased from national firms. Based on these weighted results, we determined that SNFs purchase, on average, the following portions of contracted professional services inside their local labor market:
- 78 percent of legal services.
- 86 percent of accounting and auditing services.
- 89 percent of architectural, engineering services.
- 87 percent of management consulting services.
Together, these four categories represent 3.6 percentage points of the total costs for the proposed 2022-based SNF market basket. We applied the percentages from this special survey to their respective SNF market basket weights to separate them into labor-related and nonlabor-related costs. As a result, we are designating 2.8 of the 3.6 percentage points total to the labor-related share, with the remaining 0.8 percentage point categorized as nonlabor-related.
In addition to the professional services as previously listed, for the 2022-based SNF market basket, we proposed to allocate a proportion of the Home Office/Related Organization Contract Labor cost weight, calculated using the Medicare cost reports as previously stated, into the Professional Fees: Labor-Related and Professional Fees: Nonlabor-Related cost categories. We proposed to classify these expenses as labor-related and nonlabor-related as many facilities are not located in the same geographic area as their home office, and, therefore, do not meet our definition for the labor-related share that requires the services to be purchased in the local labor market.
Similar to the 2018-based SNF market basket, we proposed for the 2022-based SNF market basket to use the Medicare cost reports for SNFs to determine the home office labor-related percentages. The Medicare cost report requires a SNF to report information regarding its home office provider. Using information on the Medicare cost report, we compared the location of the SNF with the location of the SNF's home office. We proposed to classify a SNF with a home office located in their respective labor market if the SNF and its home office are located in the same Metropolitan Statistical Area (MSA). Then we determined the proportion of the Home Office/Related Organization Contract Labor cost weight that should be allocated to the labor-related share based on the percent of total Home Office/Related Organization Contract Labor costs for those SNFs that had home offices located in their respective local labor markets of total Home Office/Related Organization Contract Labor costs for SNFs with a home office. We determined a SNF's and its home office's MSA using their zip code information from the Medicare cost report.
Using this methodology, we determined that 25 percent of SNFs' Home Office/Related Organization Contract Labor costs were for home offices located in their respective local labor markets. Therefore, we proposed to allocate 25 percent of the Home Office/Related Organization Contract Labor cost weight (0.1 percentage point = 0.6 percent × 25 percent) to the Professional Fees: Labor-Related cost weight and 75 percent of the Home Office/Related Organization Contract Labor cost weight to the Professional Fees: Nonlabor-Related cost weight (0.4 percentage point = 0.6 percent × 75 percent). The 2018-based SNF market basket used a similar methodology for allocating the Home Office/Related Organization Contract Labor cost weight to the labor-related share.
In summary, based on the two allocations mentioned earlier, we proposed to apportion 2.9 percentage points into the Professional Fees: Labor-Related cost category consisting of the Professional Fees (2.8 percentage points) and Home Office/Related Organization Contract Labor (0.1 percentage point) cost weights. This amount was added to the portion of professional fees that we already identified as labor-related using the I-O data such as contracted advertising and marketing costs (approximately 0.6 percentage point of total costs) resulting in a Professional Fees: Labor-Related cost weight of 3.6 percent.
Based on IHS Global, Inc.'s fourth-quarter 2023 forecast with historical data through the third quarter of 2023, we proposed a FY 2025 labor-related share of 71.9 percent.
Comment: One commenter did not support any increases in the labor-related share because facilities with a wage index less than 1.0 will suffer financially from a rise in the labor-related share. They stated that across the country, there is a growing disparity between the high-wage and low-wage States.
Response: We appreciate the commenter's concern. However, for this final rule, we are finalizing our proposal to rebase the SNF market basket to reflect a 2022 base year so that we can incorporate more recent data on SNF cost structures. In addition, we calculate a labor-related share based on the relative importance of labor-related cost categories, to account for historical and projected price changes between the base year and the payment year (FY 2025 in this rule). The price proxies for the different cost categories in the market basket do not necessarily change at the same rate, and the relative importance measure captures these changes. We recognize that a change in the labor-related share can have differential impacts for providers, but we believe it is important to continue to update the labor-related share to reflect the current SNF cost environment.
As was stated in the FY 2025 SNF PPS proposed rule (89 FR 23451), if more recent data subsequently became available, we would use such data, if appropriate, to determine the FY 2025 SNF labor-related share relative importance. Accordingly, based on IGI's second-quarter 2024 forecast with historical data through the first quarter of 2024, the labor-related share for FY 2025 based on the finalized 2022-based SNF market basket is 72.0 percent.
Table 20 compares the FY 2025 labor-related share based on the 2022-based SNF market basket relative importance and the FY 2024 labor-related share based on the 2018-based SNF market basket relative importance as finalized in the FY 2024 SNF final rule (88 FR 53213).
The FY 2025 SNF labor-related share is 0.9 percentage point higher than the FY 2024 SNF labor-related share (based on the 2018-based SNF market basket). The higher labor-related share is primarily due to incorporating the 2022 Medicare cost report data, which resulted in a higher Compensation cost weight, as well as higher relative importance of the Capital cost category.
5. FY 2025 Market Basket Percentage Increase for the SNF PPS Update
As discussed previously in this rule, beginning with the FY 2025 SNF PPS update, we are adopting the 2022-based SNF market basket as the appropriate market basket of goods and services for the SNF PPS. Consistent with historical practice, we estimate the market basket update for the SNF PPS based on IHS Global Inc.'s (IGI) forecast. IGI is a nationally recognized economic and financial forecasting firm with which CMS contracts to forecast the components of the market baskets and total factor productivity (TFP).
Based on IGI's fourth-quarter 2023 forecast with historical data through the third quarter of 2023, the proposed 2022-based SNF market basket update for FY 2025 was estimated to be 2.8 percent—which was 0.1 percentage point lower than the FY 2025 percent change of the 2018-based SNF market basket. We are also proposed that if more recent data subsequently became available (for example, a more recent estimate of the market basket and/or the TFP), we would use such data, if appropriate, to determine the FY 2025 SNF market basket percentage increase, labor-related share, forecast error adjustment, or productivity adjustment in the SNF PPS final rule. Accordingly, based on IGI's second-quarter 2024 forecast with historical data through the first quarter of 2024, the most recent estimate of the 2022-based SNF market basket percentage increase for FY 2025 is 3.0 percent.
Table 21 compares the 2022-based SNF market basket and the 2018-based SNF market basket percent changes. While there are slight differences of up to 0.2 percentage point in certain years, there is no difference in the average growth rates between the two market baskets in the historical period (FY 2020-FY 2023) and a 0.1 percentage point difference in the forecast period (FY 2024-FY 2026) when rounded to one decimal place.
B. Changes to SNF PPS Wage Index
1. Core-Based Statistical Areas (CBSAs) for the FY 2025 SNF PPS Wage Index
a. Background
Section 1888(e)(4)(G)(ii) of the Act requires that we adjust the Federal rates to account for differences in area wage levels, using a wage index that the Secretary determines appropriate. Since the inception of the SNF PPS, we have used hospital inpatient wage data in developing a wage index to be applied to SNFs. We proposed to continue this practice for FY 2025, as we continue to believe that in the absence of SNF-specific wage data, using the hospital inpatient wage index data is appropriate and reasonable for the SNF PPS. As explained in the update notice for FY 2005 (69 FR 45786), the SNF PPS does not use the hospital area wage index's occupational mix adjustment, as this adjustment serves specifically to define the occupational categories more clearly in a hospital setting; moreover, the collection of the occupational wage data under the IPPS also excludes any wage data related to SNFs. Therefore, we believe that using the updated wage data exclusive of the occupational mix adjustment continues to be appropriate for SNF payments. As in previous years, we would continue to use, as the basis for the SNF PPS wage index, the IPPS hospital wage data, unadjusted for occupational mix, without taking into account geographic reclassifications under section 1886(d)(8) and (d)(10) of the Act, and without applying the rural floor under section 4410 of the BBA 1997 and the outmigration adjustment under section 1886(d)(13) of the Act. For FY 2025, the updated wage data are for hospital cost reporting periods beginning on or after October 1, 2020, and before October 1, 2021 (FY 2021 cost report data).
The applicable SNF PPS wage index value is assigned to a SNF on the basis of the labor market area in which the SNF is geographically located. In the SNF PPS final rule for FY 2006 (70 FR 45026, August 4, 2005), we adopted the changes discussed in OMB Bulletin No. 03-04 (June 6, 2003), which announced revised definitions for Metropolitan Statistical Area (MSA) and the creation of micropolitan statistical areas and combined statistical areas. In adopting the Core-Based Statistical Areas (CBSA) geographic designations, we provided for a 1-year transition in FY 2006 with a blended wage index for all providers. For FY 2006, the wage index for each provider consisted of a blend of 50 percent of the FY 2006 MSA-based wage index and 50 percent of the FY 2006 CBSA-based wage index (both using FY 2002 hospital data). We referred to the blended wage index as the FY 2006 SNF PPS transition wage index. As discussed in the SNF PPS final rule for FY 2006 (70 FR 45041), since the expiration of this 1-year transition on September 30, 2006, we have used the full CBSA-based wage index values.
In the FY 2015 SNF PPS final rule (79 FR 45644 through 45646), we finalized changes to the SNF PPS wage index based on the newest OMB delineations, as described in OMB Bulletin No. 13-01, beginning in FY 2015, including a 1-year transition with a blended wage index for FY 2015. OMB Bulletin No. 13-01 established revised delineations for MSAs, Micropolitan Statistical Areas, and Combined Statistical Areas in the United States and Puerto Rico based on the 2010 Census, and provided guidance on the use of the delineations of these statistical areas using standards published in the June 28, 2010 Federal Register (75 FR 37246 through 37252). Subsequently, on July 15, 2015, OMB issued OMB Bulletin No. 15-01, which provided minor updates to and superseded OMB Bulletin No. 13-01 that was issued on February 28, 2013. The attachment to OMB Bulletin No. 15-01 provided detailed information on the update to statistical areas since February 28, 2013. The updates provided in OMB Bulletin No. 15-01 were based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to Census Bureau population estimates for July 1, 2012 and July 1, 2013. In addition, on August 15, 2017, OMB issued Bulletin No. 17-01 which announced a new urban CBSA, Twin Falls, Idaho (CBSA 46300). As we previously stated in the FY 2008 SNF PPS proposed and final rules (72 FR 25538 through 25539, and 72 FR 43423), and as we noted in the proposed rule, this and all subsequent SNF PPS rules and notices are considered to incorporate any updates and revisions set forth in the most recent OMB bulletin that applies to the hospital wage data used to determine the current SNF PPS wage index.
On April 10, 2018, OMB issued OMB Bulletin No. 18-03 which superseded the August 15, 2017 OMB Bulletin No. 17-01. Subsequently, on September 14, 2018, OMB issued OMB Bulletin No. 18-04, which superseded the April 10, 2018 OMB Bulletin No. 18-03. These bulletins established revised delineations for MSAs, Micropolitan Statistical Areas, and Combined Statistical Areas, and provided guidance on the use of the delineations of these statistical areas. A copy of OMB Bulletin No. 18-04, may be obtained at https://www.whitehouse.gov/wp-content/uploads/2018/09/Bulletin-18-04.pdf. While OMB Bulletin No. 18-04 is not based on new census data, it includes some material changes to the OMB statistical area delineations, including some new CBSAs, urban counties that would become rural, rural counties that would become urban, and existing CBSAs that would be split apart. OMB issued further revised CBSA delineations in OMB Bulletin No. 20-01, on March 6, 2020 (available on the web at https://www.whitehouse.gov/wp-content/uploads/2020/03/Bulletin-20-01.pdf). However, we determined that the changes in OMB Bulletin No. 20-01 do not impact the CBSA-based labor market area delineations adopted in FY 2021. Therefore, CMS did not propose to adopt the revised OMB delineations identified in OMB Bulletin No. 20-01 for FY 2022 through FY 2024.
On July 21, 2023, OMB issued OMB Bulletin No. 23-01 (available at https://www.whitehouse.gov/wp-content/uploads/2023/07/OMB-Bulletin-23-01.pdf) which updates and supersedes OMB Bulletin No. 20-01 based upon the 2020 Standards for Delineating Core Based Statistical Areas (“the 2020 Standards”) published by the Office of Management and Budget (OMB) on July 16, 2021 (86 FR 37770). OMB Bulletin No. 23-01 revised CBSA delineations which are comprised of counties and equivalent entities (for example, boroughs, a city and borough, and a municipality in Alaska, planning regions in Connecticut, parishes in Louisiana, municipios in Puerto Rico, and independent cities in Maryland, Missouri, Nevada, and Virginia). For FY 2025, we are adopting the revised OMB delineations identified in OMB Bulletin No. 23-01.
To implement these changes for the SNF PPS beginning in FY 2025, it is necessary to identify the revised labor market area delineation for each affected county and provider in the country. The revisions OMB published on July 21, 2023 contain a number of significant changes. For example, under the revised OMB delineations, there would be new CBSAs, urban counties that would become rural, rural counties that would become urban, and existing CBSAs that would split apart. We discussed these changes in more detail in the proposed rule.
b. Implementation of Revised Labor Market Area Delineations
We typically delay implementing OMB labor market area delineations to allow for sufficient time to assess the new changes. For example, as discussed in the FY 2014 SNF PPS proposed rule (78 FR 26448) and final rule (78 FR 47952), we delayed implementing the revised OMB statistical area delineations described in OMB Bulletin No. 13-01 to allow for sufficient time to assess the new changes. We believe it is important for the SNF PPS to use the latest labor market area delineations available as soon as is reasonably possible to maintain a more accurate and up-to-date payment system that reflects the reality of population shifts and labor market conditions. We further believe that using the delineations reflected in OMB Bulletin No. 23-01 would increase the integrity of the SNF PPS wage index system by creating a more accurate representation of geographic variations in wage levels. We have reviewed our findings and impacts relating to the revised OMB delineations set forth in OMB Bulletin No. 23-01 and find no compelling reason to further delay implementation. Because we believe we have broad authority under section 1888(e)(4)(G)(ii) of the Act to determine the labor market areas used for the SNF PPS wage index, and because we believe the delineations reflected in OMB Bulletin No. 23-01 better reflect the local economies and wage levels of the areas in which hospitals are currently located, we proposed to implement the revised OMB delineations as described in the July 21, 2023 OMB Bulletin No. 23-01, for the SNF PPS wage index effective beginning in FY 2025. In addition, we will apply the permanent 5 percent cap policy in FY 2025 on decreases in a hospital's wage index compared to its wage index for the prior fiscal year (FY 2024) to assist providers in adapting to the revised OMB delineations (if we finalize the implementation of such delineations for the SNF PPS wage index beginning in FY 2025). This policy is discussed in more detail in the proposed rule. We solicited comments on these proposals.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: Commenters generally support the proposed policies for FY 2025. One commenter stated that it “seems to strike a balance between fairly compensating SNFs, promoting quality care, and enhancing regulatory oversight.” Another commenter appreciates that CMS is not requiring the commitment resources needed to do cost report audits at this time. However, a number of these commenters also recommend CMS continue to reform the wage index policies. These recommendations included suggestions such as modifying the current methodology by developing a reclassification policy similar to the hospital wage index reclassification policy or developing a SNF-specific wage index.
Response: We appreciate the commenters' support of the wage index proposed policies for FY 2025. In the absence of a SNF-specific wage index, we continue to believe the use of the pre-reclassified and pre-floor hospital wage data (without the occupational mix adjustment) continue to be an appropriate and reasonable proxy for the SNF PPS. For a detailed discussion of the rationale for our current wage index policies and for responses to these recurring comments, we refer readers to the FY 2024 SNF PPS final rule (88 FR 53211 through 53215) and the FY 2016 SNF PPS final rule (80 FR 46401 through 46402).
Comment: One commenter, who disagrees with the proposed delineation changes, specifically expressed concerns with the wage index decrease of both Rock County, Minnesota, and McHenry County, North Dakota. Both counties will transition from rural to urban designation and in turn will experience slightly over a 12 percent decrease from FY 2024 to FY 2025. Due to the decline in wage index, the commenter strongly requests CMS to review the wage index data for Trinity Health (the only rural PPS hospital in North Dakota prior to the proposed designation change).
Response: We understand that some CBSAs may experience a wage index decline compared to the previous fiscal year. For North Dakota, our investigation discovered the wage data for Trinity Health (provider 350006) was audited in FY 2025 with no issues reported. The average hourly wage reported for Trinity Health declined 7 percent since FY 2024. For the purposes of the SNF PPS, if a SNF (not hospital) experience a rural or urban redesignation due to the proposed delineation changes for FY 2025 and their wage index resulted in decline since FY 2024, the 5 percent cap policy will be applied. Therefore, we continue to believe that the 5 percent cap policy will mitigate any significant decreases a SNF may experience due to the revised OMB delineations. Additional details on the wage index transition policy for FY 2025 is discussed further below in this section. After consideration of public comments, we are finalizing our proposal regarding the implementation of the revised labor market area delineations for FY 2025.
(1) Micropolitan Statistical Areas
As discussed in the FY 2006 SNF PPS proposed rule (70 FR 29093 through 29094) and final rule (70 FR 45041), we considered how to use the Micropolitan Statistical Area definitions in the calculation of the wage index. OMB defines a “Micropolitan Statistical Area” as a CBSA “associated with at least one urban cluster that has a population of at least 10,000, but less than 50,000” (75 FR 37252). We refer to these as Micropolitan Areas. After extensive impact analysis, consistent with the treatment of these areas under the IPPS as discussed in the FY 2005 IPPS final rule (69 FR 49029 through 49032), we determined the best course of action would be to treat Micropolitan Areas as “rural” and include them in the calculation of each State's SNF PPS rural wage index (see 70 FR 29094 and 70 FR 45040 through 45041).
Thus, the SNF PPS statewide rural wage index is determined using IPPS hospital data from hospitals located in non-MSA areas, and the statewide rural wage index is assigned to SNFs located in those areas. Because Micropolitan Areas tend to encompass smaller population centers and contain fewer hospitals than MSAs, we determined that if Micropolitan Areas were to be treated as separate labor market areas, the SNF PPS wage index would have included significantly more single-provider labor market areas. As we explained in the FY 2006 SNF PPS proposed rule (70 FR 29094), recognizing Micropolitan Areas as independent labor markets would generally increase the potential for dramatic shifts in year-to-year wage index values because a single hospital (or group of hospitals) could have a disproportionate effect on the wage index of an area. Dramatic shifts in an area's wage index from year-to-year are problematic and create instability in the payment levels from year-to-year, which could make fiscal planning for SNFs difficult if we adopted this approach. For these reasons, we adopted a policy to include Micropolitan Areas in the State's rural wage area for purposes of the SNF PPS wage index and have continued this policy through the present.
We believe that the best course of action would be to continue the policy established in the FY 2006 SNF PPS final rule and include Micropolitan Areas in each State's rural wage index. These areas continue to be defined as having relatively small urban cores (populations of 10,000 to 49,999). We do not believe it would be appropriate to calculate a separate wage index for areas that typically may include only a few hospitals for the reasons discussed in the FY 2006 SNF PPS proposed rule, and as discussed earlier. Therefore, in conjunction with our implementing of the revised OMB labor market delineations beginning in FY 2025 and consistent with the treatment of Micropolitan Areas under the IPPS, we proposed to continue to treat Micropolitan Areas as “rural” and to include Micropolitan Areas in the calculation of the State's rural wage index.
(2) Urban Counties That Would Become Rural Under the Revised OMB Delineations
As previously discussed, we proposed to implement the new OMB statistical area delineations (based upon the 2020 decennial Census data) beginning in FY 2025 for the SNF PPS wage index. Our analysis shows that a total of 54 counties (and county equivalents) that are currently considered part of an urban CBSA will be considered located in a rural area, for SNF PPS payment beginning in FY 2025, when we adopt the new OMB delineations. Table 22 lists the 54 urban counties that will be rural when we finalized our proposal to implement the new OMB delineations.
We proposed that, for purposes of determining the wage index under the SNF PPS, the wage data for all hospitals located in the counties listed in Table 22 would be considered rural when calculating their respective State's rural wage index under the SNF PPS. We recognize that rural areas typically have lower area wage index values than urban areas, and SNFs located in these counties may experience a negative impact in their SNF PPS payment due to the adoption of the revised OMB delineations. Furthermore, for SNF providers currently located in an urban county that will be considered rural when this proposal will be finalized, we will utilize the rural unadjusted per diem rates, found in Table 14, as the basis for determining payment rates for these facilities beginning on October 1, 2024.
(3) Rural Counties That Would Become Urban Under the Revised OMB Delineations
As previously discussed, we proposed to implement the revised OMB statistical area delineations based upon OMB Bulletin No. 18-04 beginning in FY 2025. Analysis of these OMB statistical area delineations shows that a total of 54 counties (and county equivalents) that are currently located in rural areas will be located in urban areas when we finalize our proposal to implement the revised OMB delineations.
Table 23 lists the 54 rural counties that will be urban when we finalize this proposal.
We proposed that, for purposes of calculating the area wage index under the SNF PPS, the wage data for hospitals located in the counties listed in Table 23 will be included in their new respective urban CBSAs. Typically, SNFs located in an urban area will receive a wage index value higher than or equal to SNFs located in their State's rural area. Furthermore, for SNFs currently located in a rural county that will be considered urban when this proposal be finalized, we will utilize the urban unadjusted per diem rates found in Table 23, as the basis for determining the payment rates for these facilities beginning October 1, 2024.
(4) Urban Counties That Would Move to a Different Urban CBSA Under the Revised OMB Delineations
In addition to rural counties becoming urban and urban counties becoming rural, several urban counties will shift from one urban CBSA to another urban CBSA under adoption of the new OMB delineations. In other cases, when we adopt the new OMB delineations, counties will shift between existing and new CBSAs, changing the constituent makeup of the CBSAs.
In one type of change, an entire CBSA will be subsumed by another CBSA. For example, CBSA 31460 (Madera, CA) currently is a single county (Madera, CA) CBSA. Madera County will be a part of CBSA 23420 (Fresno, CA) under the new OMB delineations.
In another type of change, some CBSAs have counties that would split off to become part of, or to form, entirely new labor market areas. For example, CBSA 29404 (Lake County-Kenosha County, IL-WI) currently is comprised of two counties (Lake County, IL, and Kenosha County, WI). Under the new OMB delineations, Kenosha county will split off and form the new CBSA 28450 (Kenosha, WI), while Lake county would remain in CBSA 29404.
Finally, in some cases, a CBSA will lose counties to another existing CBSA when we adopt the new OMB delineations. For example, Meade County, KY, will move from CBSA 21060 (Elizabethtown-Fort Knox, KY) to CBSA 31140 (Louisville/Jefferson County, KY-IN). CBSA 21060 will still exist in the new labor market delineations with fewer constituent counties. Table 24 lists the urban counties that will move from one urban CBSA to another urban CBSA under the new OMB delineations.
If providers located in these counties move from one CBSA to another under the new OMB delineations, there may be impacts, both negative and positive, upon their specific wage index values.
In other cases, adopting the revised OMB delineations will involve a change only in CBSA name and/or number, while the CBSA continues to encompass the same constituent counties. For example, CBSA 19430 (Dayton-Kettering, OH) will experience a change to its name and become CBSA 19430 (Dayton-Kettering-Beavercreek, OH), while all of its three constituent counties will remain the same. We consider these changes (where only the CBSA name and/or number will change) to be inconsequential changes with respect to the SNF PPS wage index. Table 25 sets forth a list of such CBSAs where there will be a change in CBSA name and/or number only when we adopt the revised OMB delineations.
5. Change to County-Equivalents in the State of Connecticut
The June 6, 2022 Census Bureau Notice (87 FR 34235-34240), OMB Bulletin No. 23-01 replaced the 8 counties in Connecticut with 9 new “Planning Regions.” Planning regions now serve as county-equivalents within the CBSA system. We proposed to adopt the planning regions as county equivalents for wage index purposes. We believe it is necessary to adopt this migration from counties to planning region county-equivalents in order to maintain consistency with OMB updates. As outlined in the proposed rule, we are providing the following crosswalk with the current and proposed FIPS county and county-equivalent codes and CBSA assignments.
2. Transition Policy for FY 2025 Wage Index Changes
Overall, we believe that implementing the new OMB delineations will result in wage index values being more representative of the actual costs of labor in a given area. We recognize that some SNFs (43 percent) will experience decreases in their area wage index values as a result of this change, though less than 1 percent of providers will experience a significant decrease (that is, greater than 5 percent) in their area wage index value. We also realize that many SNFs (57 percent) will have higher area wage index values after adopting the revised OMB delineations.
CMS recognizes that SNFs in certain areas may experience reduced payment due to the adoption of the revised OMB delineations and has finalized transition policies to mitigate negative financial impacts and provide stability to year-to-year wage index variations. In FY 2023, the 5 percent cap policy was made permanent for all SNFs. This 5 percent cap on reductions policy is discussed in further detail in FY 2023 final rule at 87 FR 47521 through 47523. It is CMS' long held opinion that revised labor market delineations should be adopted as soon as is possible to maintain the integrity the wage index system. We believe the 5 percent cap policy will sufficiently mitigate significant disruptive financial impacts on SNFs negatively affected by the adoption of the revised OMB delineations. We do not believe any additional transition is necessary considering that the current cap on wage index decreases, which was not in place when implementing prior decennial census updates in FY 2006 and FY 2015, ensures that a SNF's wage index will not be less than 95 percent of its final wage index for the prior year.
Furthermore, consistent with the requirement at section 1888(e)(4)(G)(ii) of the Act that wage index adjustments must be made in a budget neutral manner, the applied 5 percent cap on the decrease in an SNF's wage index will not result in any change in estimated aggregate SNF PPS payments by applying a budget neutrality factor to the unadjusted Federal per diem rates. The methodology for calculating this budget neutrality factor is outlined in section III.D of the proposed rule.
We solicited comments on our proposed implementation of revised labor market area delineations. The proposed wage index applicable to FY 2025 is set forth in Table A and B available on the CMS website at https://cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/WageIndex.html.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: Several commenters support the existing 5 percent permanent cap policy but raised concerns on varying impacts it has on different providers. A commenter recommends that the 5 percent cap be applied in a non-budget neutral manner. Another commenter suggest that CMS apply a 1-year transition period to allow time to study the impact of the delineation changes. A commenter suggest CMS lower the cap amount to mitigate changes caused by revisions to the CBSA delineations.
Response: We appreciate the commenters' support of the permanent 5 percent cap on wage index decreases policy. When the permanent 5 percent cap policy was established in FY 2023, our provider level impact analysis determined approximately 97 percent of SNFs would experience a wage index change within 5 percent. Therefore, we believe applying a 5-percent cap on all wage index decreases each year, regardless of the reason for the decrease, would effectively mitigate instability in SNF PPS payments due to any significant wage index decreases that may affect providers in any year. As discussed earlier in this section, it is CMS' long held opinion that revised labor market delineations should be adopted as soon as is possible to maintain the integrity the wage index system. We believe the 5 percent cap policy will sufficiently mitigate significant disruptive financial impacts on SNFs negatively affected by the proposed adoption of the revised OMB delineations. As for budget neutrality, we do not believe that the permanent 5 percent cap policy for the SNF wage index should be applied in a non-budget-neutral manner. As a matter of fact, the statute at section 1888(e)(4)(G)(ii) of the Act requires that adjustments for geographic variations in labor costs for a FY are made in a budget-neutral manner. We refer readers to the FY 2023 SNF PPS final rule (87 FR 47521 through 47523) for a detailed discussion and for responses to these and other comments relating to the wage index cap policy.
After consideration of public comments, we are finalizing our proposal regarding the wage index adjustment for FY 2025.
C. Technical Updates to the PDPM ICD-10 Mappings
1. Background
In the FY 2019 SNF PPS final rule (83 FR 39162), we finalized the implementation of the Patient Driven Payment Model (PDPM), effective October 1, 2019. The PDPM utilizes the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM, hereafter referred to as ICD-10) codes in several ways, including using the patient's primary diagnosis to assign patients to clinical categories under several PDPM components, specifically the PT, OT, SLP, and NTA components. While other ICD-10 codes may be reported as secondary diagnoses and designated as additional comorbidities, the PDPM does not use secondary diagnoses to assign patients to clinical categories. The PDPM ICD-10 code to clinical category mapping, ICD-10 code to SLP comorbidity mapping, and ICD-10 code to NTA comorbidity mapping (hereafter collectively referred to as the PDPM ICD-10 code mappings) are available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/PDPM.
In the FY 2020 SNF PPS final rule (84 FR 38750), we outlined the process by which we maintain and update the PDPM ICD-10 code mappings, as well as the SNF Grouper software and other such products related to patient classification and billing, to ensure that they reflect the most up to date codes. Beginning with the updates for FY 2020, we apply non-substantive changes to the PDPM ICD-10 code mappings through a sub-regulatory process consisting of posting the updated PDPM ICD-10 code mappings on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/PDPM. Such nonsubstantive changes are limited to those specific changes that are necessary to maintain consistency with the most current PDPM ICD-10 code mappings.
On the other hand, substantive changes that go beyond the intention of maintaining consistency with the most current PDPM ICD-10 code mappings, such as changes to the assignment of a code to a clinical category or comorbidity list, would be through notice and comment rulemaking because they are changes that affect policy. We noted in the proposed rule that in the case of any diagnoses that are either currently mapped to Return to Provider or that we are finalizing to classify into this category, this is not intended to reflect any judgment on the importance of recognizing and treating these conditions. Rather, we believe that there are more specific or appropriate diagnoses that would better serve as the primary diagnosis for a Part-A covered SNF stay.
2. Clinical Category Changes for New ICD-10 Codes for FY 2025
Each year, we review the clinical category assigned to new ICD-10 diagnosis codes and proposed changing the assignment to another clinical category if warranted. This year, we proposed changing the clinical category assignment for the following four new codes that were effective on October 1, 2023.
- E88.10Metabolic Syndrome was initially mapped to the clinical category of Medical Management. The National Institutes of Health (NIH) defines metabolic syndrome as the presence of at least three of the following traits: Large waist, elevated triglyceride levels, reduced high-density lipoprotein (HDL) cholesterol, increased blood pressure, and/or elevated fasting blood glucose. Metabolic syndrome is a cluster of metabolic risk factors for cardiovascular diseases and type 2 diabetes mellitus. The root causes of metabolic syndrome are overweight/obesity, physical inactivity, and genetic factors. Given this, treatment for Metabolic Syndrome typically occurs outside of a Part A SNF stay and we do not believe it would serve appropriately as the primary diagnosis for a Part A-covered SNF stay. For this reason, we proposed to change the mapping of this code from Medical Management to the clinical category of Return to Provider.
- E88.811Insulin Resistance Syndrome, Type A was initially mapped to the clinical category of Medical Management. Type A insulin resistance syndrome (TAIRS) is a rare disorder characterized by severe insulin resistance due to defects in insulin receptor signaling and treatment typically occurs outside of a Part A SNF stay. For this reason, we proposed to change the mapping of this code from Medical Management to the clinical category of Return to Provider.
- E88.818Other Insulin Resistance was initially mapped to the clinical category of Medical Management. Other Insulin Resistance is used to specify a medical diagnosis of other insulin resistance such as Insulin resistance, Type B. Treatment typically occurs outside of a Part A SNF stay. For this reason, we proposed to change the mapping of this code from Medical Management to the clinical category of Return to Provider.
- E88.819Insulin Resistance, Unspecified was initially mapped to the clinical category of Medical Management and is utilized to indicate when a specific type of insulin resistance has not been specifically identified. Treatment typically occurs outside of a Part A SNF stay. For this reason, we proposed to change the mapping of this code from Medical Management to the clinical category of Return to Provider.
We solicited comments on the proposed substantive changes to the PDPM ICD-10 code mappings outlined in this section, as well as comments on additional substantive and non-substantive changes that commenters believe are necessary.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: Several commenters supported the proposed reclassification of the PDPM ICD-10 mappings of E88.10 Metabolic Syndrome, E88.811 Insulin Resistance Syndrome, E88.818 Other Insulin Resistance, and E88.819 Insulin Resistance, Unspecified from Medical Management to the Return to Provider (RTP) category. Commenters agreed these mapping changes would improve billing accuracy, promote more appropriate diagnoses for SNF stays, and ultimately improve patient care.
Response: We appreciate the support for these proposed ICD-10 mapping changes.
Comment: One commenter stated CMS should reconsider mapping ICD-10 code M62.81, Muscle Weakness (Generalized) from RTP to alternative category and be used as a primary diagnosis.
Response: We considered this request and, as noted in 87 FR 47524, continue to believe, as discussed in the FY 2023 SNF PPS final rule (87 FR 47524), that M62.81 Muscle Weakness (Generalized) is nonspecific and if the original condition is resolved, but the resulting muscle weakness persists because of the known original diagnosis, there are more specific codes that exist that would account for why the muscle weakness is on-going. Many musculoskeletal conditions are the result of a previous injury or trauma to a site or are recurrent conditions. This symptom, without any specification of the etiology or severity, is not a reason for daily skilled care in a SNF. Patients with Muscle Weakness (Generalized) should obtain a more specific diagnosis causing the generalized muscle weakness. The specific diagnosis should be used to develop an appropriate care plan for the patient.
Comment: Several commenters recommended that CMS consider additional changes to the ICD-10 mappings. These include additional dysphagia code mappings for the Speech Language Pathology component, changes to how PDPM classifies dialysis patients, and adding codes that will reflect complications related to the GI devices.
Response: We appreciate the comments and, to the extent that these changes represent substantive changes to the ICD-10 code mappings, we will consider these comments for future rulemaking.
After consideration of public comments, we are finalizing the changes described above, as proposed.
D. Request for Information: Update to PDPM Non-Therapy Ancillary Component
1. Background
In the FY 2019 SNF PPS final rule (83 FR 39162), we finalized the implementation of the PDPM, effective October 1, 2019. Under the PDPM, payment is determined through the combination of six payment components. Five of the components (PT, OT, SLP, NTA, and nursing) are case-mix adjusted. Additionally, there is a non-case-mix adjusted component to cover utilization of SNF resources that do not vary according to patient characteristics.
The NTA component utilizes a comorbidity score to assign the patient to an NTA component case-mix group, which is determined by the presence of conditions or the use of extensive services (henceforth also referred to as comorbidities) that were found to be correlated with increases in NTA costs for SNF patients. The presence of these comorbidities is reported by providers on certain items of the Minimum Data Set (MDS) resident assessment, with some comorbidities being identified by ICD-10-CM diagnosis codes (hereafter referred to as ICD-10 codes) that are coded in Item I8000 of the MDS. MDS Item I8000 is an open-ended item on the MDS assessment where the provider can fill in additional active diagnoses for the patient that are either not explicitly on the MDS, or are more severe or specific diagnoses, in the form of ICD-10 codes. For conditions and extensive services where the source is indicated as MDS Item I8000, CMS posts an NTA comorbidity to ICD-10 mapping, available at https://www.cms.gov/medicare/payment/prospective-payment-systems/skilled-nursing-facility-snf/patient-driven-model, that provides a crosswalk between the listed condition and the ICD-10 codes that may be coded to qualify that condition to serve as part of the patient's NTA classification.
During the development of PDPM, CMS identified a list of 50 conditions and extensive services that were associated with increases in NTA costs. Each of the 50 comorbidities used under PDPM for NTA classification is assigned a certain number of points based on its relative costliness. To determine the patient's NTA comorbidity score, a provider would identify all the comorbidities for which a patient would qualify and then add the points for each comorbidity together. The resulting sum represents the patient's NTA comorbidity score, which is then used to classify the patient into an NTA component classification group. More information about the creation of the NTA component scoring method can be found in section 3.7 of the SNF PDPM Technical Report, available at https://www.cms.gov/medicare/payment/prospective-payment-systems/skilled-nursing-facility-snf/pps-model-research.
In response to feedback from interested parties, CMS stated in the FY 2019 SNF PPS final rule that we would consider revisiting both the list of comorbidities used under the NTA component and the points assigned to each condition or extensive service based on changes in the patient population and care practices over time (83 FR 39224). Accordingly, in the FY 2025 SNF PPS proposed rule, we released a request for information (RFI) soliciting comment on the methodology CMS is currently considering for updating the NTA component (89 FR 23459 through 89 FR 23461).
2. Updates to the Study Population and Methodology
We are considering several changes to the NTA study population as a foundation upon which to update the NTA component. First, we are considering updating the years used for data corresponding to Medicare Part A SNF stays, including claims, assessments, and cost reports. To develop PDPM, CMS used a study population of Medicare Part A SNF stays with admissions from FY 2014 through FY 2017 (see FY 2019 SNF PPS final rule, 83 FR 39220). This methodology is described in more detail in section 3.2.1 of the SNF PDPM technical report, available at https://www.cms.gov/medicare/payment/prospective-payment-systems/skilled-nursing-facility-snf/pps-model-research. The updated study population will instead use Medicare Part A SNF stays with admissions from FY 2019 through FY 2022. However, as discussed in the FY 2023 SNF PPS final rule (87 FR 47526 through 47528), data from much of this time period was affected by the national COVID-19 PHE with significant impacts on nursing homes. We are therefore considering using the same subset population used for the PDPM parity adjustment recalibration by excluding stays with either a COVID-19 diagnosis or stays using a COVID-19 PHE-related modification under section 1812(f) of the Act.
Next, we are considering making certain methodological changes to reflect more accurate and reliable coding of NTA conditions and extensive services on SNF Part A claims and the MDS after PDPM implementation. We had taken a broad approach when creating the initial list of conditions and services used under the NTA component to predict what NTA coding practices would be after PDPM implementation, given the absence of analogous data in the previous Resource Utilization Groups, Version IV (RUG-IV) payment model. The initial list of comorbidities used under the NTA component was therefore created using data from a variety of different sources, including using Medicare inpatient, outpatient, and Part B claims to identify the presence of condition categories from the Medicare Parts C and D risk adjustment models (hereafter referred to as CCs and RxCCs, respectively). More information about this methodology can be found in section 3.7 of the SNF PDPM Technical Report, available at https://www.cms.gov/medicare/payment/prospective-payment-systems/skilled-nursing-facility-snf/pps-model-research. Given that we now have several years of post-PDPM implementation data, we believe it would more accurately reflect the coding of conditions and extensive services under PDPM to rely exclusively upon SNF PPS Part A claims and the MDS. We are therefore considering updating the methodology to only utilize SNF Part A claims and the MDS, and not claim types from other Medicare settings.
Additionally, we are considering modifying the overlap methodology to rely more upon the MDS items that use a checkbox to record the presence of conditions and extensive services whenever possible, while allowing for potentially more severe or specific diagnoses to be indicated on MDS Item I8000 when it would be useful for more accurate patient classification under PDPM. During the development of the NTA component, CMS included both MDS items and ICD-10 diagnoses from the Medicare Part C CCs and Part D RxCCs. Because the CCs were developed to predict utilization of Medicare Part C services, while the RxCCs were developed to predict Medicare Part D drug costs, the largest component of NTA costs, we stated in the FY 2019 SNF PPS final rule that we believed using both sources allowed us to define the conditions and extensive services potentially associated with NTA utilization more comprehensively (83 FR 39220). In cases where there was considerable overlap between an MDS item and its CC or RxCC definition, to ensure accurate estimation of statistically significant regression results, we chose the CC or RxCC definition if it had higher average NTA cost per day than the MDS item before running the final regression analysis. More information about this methodology can be found in section 3.7 of the SNF PDPM Technical Report, available at https://www.cms.gov/medicare/payment/prospective-payment-systems/skilled-nursing-facility-snf/pps-model-research.
Since the implementation of PDPM, we believe patient conditions and extensive services are now more accurately and reliably reported by providers using MDS items. We are therefore considering prioritizing the reporting of conditions on the MDS by raising the cost threshold for selecting the overlapping CC or RxCC definitions from any additional cost to 5 dollars in average NTA cost per day, which is the amount that we observe to be generally associated with a 1-point NTA increase. Specifically, since any dollar amount less than 5 dollars would render the two options indistinguishable from each other in the point assignment when comparing relative costliness, choosing MDS items over the overlapping CC or RxCC definitions will not lead to any loss of the most expensive representations of the conditions and services in the regression model.
3. Updates to Conditions and Extensive Services Used for NTA Classification
Table 27 provides the list of conditions and extensive services that would be used for NTA classification following the various changes to the methodology described in the RFI. For each comorbidity, we have also included the frequency of stays, the average NTA cost per day, the ordinary least squares (OLS) estimate of its impact on NTA costs per day, and the assigned number of points based on its relative impact on a patient's NTA costs. Conditions and extensive services with a greater impact on NTA costs were assigned more points, while those with less of an impact were assigned fewer points. More information about this methodology can be found in section 3.7 of the SNF PDPM Technical Report, available at https://www.cms.gov/medicare/payment/prospective-payment-systems/skilled-nursing-facility-snf/pps-model-research.
We solicited comments on the RFI for updates to the NTA component of PDPM. The following is a summary of the comments we received.
Commenters supported some additions and opposed some removals to the list of conditions and services used under the NTA component. Some commenters thanked CMS for the additions of rheumatoid arthritis and mobility devices for limb prosthesis. Other commenters objected to the removal of several conditions, such as proliferative diabetic retinopathy and vitreous hemorrhage, ostomy, malnutrition and at risk for malnutrition, feeding tube, infection of and open lesions on the foot, radiation, tracheostomy, pulmonary fibrosis and other chronic lung disorders, and systemic lupus erythematosus, other connective tissue disorders, and inflammatory spondylopathies.
Commenters requested that CMS consider other suggestions for the list of conditions and services used under the NTA component, such as increasing point values, adding other conditions, or not making any changes to the list. For example, some commenters objected to decreased points for parenteral IV feeding, invasive mechanical ventilator or respirator, wound infections, and HIV/AIDS. Some commenters also questioned the underlying data behind the OLS cost estimate decreases for multi-drug resistant organism and morbid obesity, even though the NTA point allocation did not change for those conditions, with some commenters requesting increased points for morbid obesity. Commenters further suggested that CMS consider adding comorbidities such as end-stage renal disease, mental health-related diagnoses such as schizophrenia and major depression, chemotherapy, end-of life prognosis, and unstageable pressure injuries with slough or eschar. One commenter objected to any changes to the current allocation of NTA points, noting that reducing points for comorbidities that are commonly admitted to SNFs, while adding points for comorbidities that are not as commonly admitted, may result in reduced payment to facilities for conditions that are frequently cared for. Similarly, another commenter stated that while adding comorbidities makes sense, removing comorbidities does not because the correlated increased cost was set by the CMS data-driven studies completed for PDPM implementation.
Many commenters specifically objected to the removal of malnutrition and at risk for malnutrition. These commenters emphasized that malnutrition is prevalent among beneficiaries in the post-acute care setting, with undiagnosed and untreated malnutrition potentially resulting in a gradual deterioration of overall health and a decline in both physical and cognitive capabilities. In turn, malnutrition can lead to extended hospital stays, increased readmission rates, a wide range of chronic health issues (commonly the development of pressure injuries, infections, decreased ability to complete activities of daily living, and frailty/fractures), and fatalities. Additionally, if malnutrition is not identified and treated early, the need and incidence for placement of an enteral feeding tube is heightened, which precipitates more risk and expense. Commenters were concerned that removing malnutrition from the list of comorbidities used under the NTA component could prevent needed resources from going to this population and reduce the importance of the role of registered dietitians, who are integral members of the patient care team. Many commenters suggested that malnutrition should increase to two NTA points while leaving at risk for malnutrition and tube feeding at one NTA point. One commenter suggested that malnutrition should become a stand-alone therapy for increased reimbursement separate from the list of conditions and services used under the NTA component.
Other commenters suggested that the criteria for defining malnutrition could be further refined, rather than being removed entirely from the list of comorbidities used under the NTA component. For example, commenters noted that registered dietitian nutritionists receive evidenced-based training to identify malnutrition using the validated Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition (ASPEN) indicators of malnutrition (AAIM) and suggested that CMS adopt the AAIM criteria in the RAI manual for MDS Item I5600 malnutrition (protein or calorie) or at risk for malnutrition. Some commenters suggested that CMS utilize the ICD-10 diagnosis code range E40 through E46 to define malnutrition and exclude at risk of malnutrition because there is no official ICD-10 diagnosis code. Many commenters suggested that CMS provide clear guidance consisting of specific examples and coding criteria in the RAI manual for malnutrition or at risk for malnutrition, which would ensure consistency and accuracy in coding practices across healthcare facilities.
We also received some comments about the data and methodology that we presented in this RFI for how CMS revised the list of comorbidities used under the NTA component. Some commenters supported updating the NTA study methodology with more recent data, while excluding those with COVID-19 diagnoses. However, other commenters stated that there was insufficient information provided in the RFI to provide meaningful and specific feedback. Commenters recommended that CMS work through potential NTA component changes in a more transparent manner, such as publishing more detailed data and considering other opportunities to gain additional feedback from interested parties. Commenters objected to the use of FY 2019 through FY 2022 data because of the COVID-19 PHE and the effects of this PHE on the SNF patient population and data collected during this time, suggesting that CMS should instead use more stable data from FY 2022 onwards with no COVID-19 related data exclusions. Some commenters recommended that CMS wait until it has at least three years of data after the end of the COVID-19 PHE. Commenters generally agreed with CMS' methodological approaches to only utilize SNF Part A claims and the MDS and not claim types from other Medicare settings that were used as a proxy to develop PDPM, but requested the flexibility to use such data in the future to include new NTA conditions as needed, such as emergent diagnoses, treatment innovations, or costs associated with certain CMS policies such as Enhanced Barrier Precautions (EBP) in nursing homes. Lastly, commenters generally agreed with modifying the overlap methodology to rely more upon MDS items that use a checkbox to record the presence of conditions and extensive services, but disagreed with CMS' method of prioritizing the MDS items by raising the cost threshold for selecting the overlapping CC or RxCC definitions (comprised of ICD-10 diagnosis codes to be entered into MDS Item I8000) from any additional cost to five dollars in average NTA cost per day.
Finally, commenters sought clarification on whether routine updates to the NTA component would be needed or beneficial in the future, as well as on the net financial impacts and if the changes would be implemented in a budget-neutral manner.
We thank commenters for their responses to the NTA RFI and we will take these comments under advisement as we consider proposed changes to the NTA component of PDPM in future rulemaking.
VII. Skilled Nursing Facility Quality Reporting Program (SNF QRP)
A. Background and Statutory Authority
The Skilled Nursing Facility Quality Reporting Program (SNF QRP) is authorized by section 1888(e)(6) of the Act, and it applies to freestanding SNFs, SNFs affiliated with acute care facilities, and all non-critical access hospital (CAH) swing-bed rural hospitals. Section 1888(e)(6)(A)(i) of the Act requires the Secretary to reduce by 2 percentage points the annual market basket percentage increase described in section 1888(e)(5)(B)(i) of the Act applicable to a SNF for a fiscal year (FY), after application of section 1888(e)(5)(B)(ii) of the Act (the productivity adjustment) and section 1888(e)(5)(B)(iii) of the Act, in the case of a SNF that does not submit data in accordance with sections 1888(e)(6)(B)(i)(II) and (III) of the Act for that FY. Section 1890A of the Act requires that the Secretary establish and follow a pre-rulemaking process, in coordination with the consensus-based entity (CBE) with a contract under section 1890(a) of the Act, to solicit input from certain groups regarding the selection of quality and efficiency measures for the SNF QRP. We have codified our program requirements in our regulations at § 413.360.
In the proposed rule, we proposed to require SNFs to collect and submit through the Minimum Data Set (MDS) four new items and modify one item on the MDS as described in section VI.C. of the proposed rule. In section VI.E.3. of the proposed rule, we proposed to adopt a similar validation process for the SNF QRP that we adopted for the SNF VBP, and to amend regulation text at § 413.360 to implement the validation process we proposed. We also sought information on future measure concepts for the SNF QRP in section VI.D. of the proposed rule.
B. General Considerations Used for the Selection of Measures for the SNF QRP
For a detailed discussion of the considerations we use for the selection of SNF QRP quality, resource use, or other measures, we refer readers to the FY 2016 SNF PPS final rule (80 FR 46429 through 46431).
1. Quality Measures Currently Adopted for the SNF QRP
The SNF QRP currently has 15 adopted measures, which are listed in Table 28. For a discussion of the factors used to evaluate whether a measure should be removed from the SNF QRP, we refer readers to § 413.360(b)(2).
We did not propose to adopt any new measures for the SNF QRP.
C. Collection of Four New Items as Standardized Patient Assessment Data Elements and Modification of One Item Collected as a Standardized Patient Assessment Data Element Beginning With the FY 2027 SNF QRP
In the proposed rule, we proposed to require SNFs to report the following four new items [2] as standardized patient assessment data elements under the social determinants of health (SDOH) category: one item for Living Situation; two items for Food; and one item for Utilities. We also proposed to modify one of the current items collected as a standardized patient assessment data element under the SDOH category (the Transportation item), as described in section VI.C.5. of the proposed rule.[3]
1. Definition of Standardized Patient Assessment Data
Section 1888(e)(6)(B)(i)(III) of the Act requires SNFs to submit standardized patient assessment data required under section 1899B(b)(1) of the Act. Section 1899B(b)(1)(A) of the Act requires post-acute care (PAC) providers to submit standardized patient assessment data under applicable reporting provisions (which, for SNFs, is the SNF QRP) with respect to the admission and discharge of an individual (and more frequently as the Secretary deems appropriate) using a standardized patient assessment instrument. Section 1899B(a)(1)(C) of the Act requires, in part, the Secretary to modify the PAC assessment instruments in order for PAC providers, including SNFs, to submit standardized patient assessment data under the Medicare program. SNFs are currently required to report standardized patient assessment data through the patient assessment instrument, referred to as the MDS. Section 1899B(b)(1)(B) of the Act describes standardized patient assessment data as data required for at least the quality measures described in section 1899B(c)(1) of the Act and that is with respect to the following categories: (1) functional status, such as mobility and self-care at admission to a PAC provider and before discharge from a PAC provider; (2) cognitive function, such as ability to express ideas and to understand, and mental status, such as depression and dementia; (3) special services, treatments, and interventions, such as need for ventilator use, dialysis, chemotherapy, central line placement, and total parenteral nutrition; (4) medical conditions and comorbidities, such as diabetes, congestive heart failure, and pressure ulcers; (5) impairments, such as incontinence and an impaired ability to hear, see, or swallow, and (6) other categories deemed necessary and appropriate by the Secretary.
2. Social Determinants of Health Collected as Standardized Patient Assessment Data Elements
Section 1899B(b)(1)(B)(vi) of the Act authorizes the Secretary to collect standardized patient assessment data elements with respect to other categories deemed necessary and appropriate. Accordingly, we finalized the creation of the SDOH category of standardized patient assessment data elements in the FY 2020 SNF PPS final rule (84 FR 38805 through 38817), and defined SDOH as the socioeconomic, cultural, and environmental circumstances in which individuals live that impact their health.[4] According to the World Health Organization, research shows that the SDOH can be more important than health care or lifestyle choices in influencing health, accounting for between 30 to 55 percent of health outcomes.[5] This is part of a growing body of research that highlights the importance of SDOH on health outcomes. Subsequent to the FY 2020 SNF PPS final rule, we expanded our definition of SDOH: SDOH are the conditions in the environments where people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.[6 7 8] This expanded definition aligns our definition of SDOH with the definition used by HHS agencies, including OASH, the Centers for Disease Control and Prevention (CDC) and the White House Office of Science and Technology Policy.[9 10] We currently collect seven items in this SDOH category of standardized patient assessment data elements: ethnicity, race, preferred language, interpreter services, health literacy, transportation, and social isolation (84 FR 38805 through 38817).[11]
In accordance with our authority under section 1899B(b)(1)(B)(vi) of the Act, we similarly finalized the creation of the SDOH category of standardized patient assessment data elements for Inpatient Rehabilitation Facilities (IRFs) in the FY 2020 IRF PPS final rule (84 FR 39149 through 39161), for Long-Term Care Hospitals (LTCHs) in the FY 2020 Inpatient Prospective Payment System (IPPS)/LTCH PPS final rule (84 FR 42577 through 84 FR 42588), and for Home Health Agencies (HHAs) in the Calendar Year (CY) 2020 HH PPS final rule (84 60597 through 60608). We also collect the same seven SDOH items in these PAC providers' respective patient assessment instruments (84 FR 39161, 84 FR 42590, and 84 FR 60610, respectively).
Access to standardized data relating to SDOH on a national level permits us to conduct periodic analyses, and to assess their appropriateness as risk adjustors or in future quality measures. Our ability to perform these analyses relies on existing data collection of SDOH items from PAC settings. We adopted these SDOH items using common standards and definitions across the four PAC providers to promote interoperable exchange of longitudinal information among these PAC providers, including SNFs, and other providers. We believe this information may facilitate coordinated care, continuity in care planning, and the discharge planning process from PAC settings.
We noted in the FY 2020 SNF PPS final rule that each of the items we were adopting at that time was identified in the 2016 National Academies of Sciences, Engineering, and Medicine (NASEM) report as impacting care use, cost and outcomes for Medicare beneficiaries (84 FR 38806). At that time, we acknowledged that other items may also be useful to understand. The SDOH items we proposed to adopt as standardized patient assessment data elements under the SDOH category in the proposed rule were also identified in the 2016 NASEM report [12] or the 2020 NASEM report [13] as impacting care use, cost and outcomes for Medicare beneficiaries. The items have the capacity to take into account treatment preferences and care goals of residents and their caregivers, to inform our understanding of resident complexity and SDOH that may affect care outcomes, and ensure that SNFs are in a position to impact them through the provision of services and supports, such as connecting residents and their caregivers with identified needs with social support programs.
Health-related social needs (HRSNs) are individual-level, adverse social conditions that negatively impact a person's health or health care,[14] and are the resulting effects of SDOH. Examples of HRSNs include lack of access to food, housing, or transportation, and have been associated with poorer health outcomes, greater use of emergency departments and hospitals, and higher health care costs.[15] Certain HRSNs can directly influence an individual's physical, psychosocial, and functional status. This is particularly true for food security, housing stability, utilities security, and access to transportation.[16]
We proposed to require SNFs to collect and submit four new items in the MDS as standardized patient assessment data elements under the SDOH category because these items would collect information not already captured by the current SDOH items. Specifically, we believe the ongoing identification of SDOH would have three significant benefits. First, promoting screening for these SDOH could serve as evidence-based building blocks for supporting healthcare providers in actualizing their commitment to address disparities that disproportionately impact underserved communities. Second, screening for SDOH improves health equity through identifying potential social needs so the SNF may address those with the resident, their caregivers, and community partners during the discharge planning process, if indicated.[17] Third, these SDOH items could support our ongoing SNF QRP initiatives by providing data with which to stratify SNF's performance on measures and in future quality measures.
Collection of additional SDOH items would permit us to continue developing the statistical tools necessary to maximize the value of Medicare data and improve the quality of care for all beneficiaries. For example, we recently developed and released the Health Equity Confidential Feedback Reports, which provided data to SNFs on whether differences in quality measure outcomes are present for their residents by dual-enrollment status and race and ethnicity.[18] We noted in the proposed rule that advancing health equity by addressing the health disparities that underlie the country's health system is one of our strategic pillars [19] and a Biden-Harris Administration priority.[20]
3. Collection of Four New Items as Standardized Patient Assessment Data Elements Beginning With the FY 2027 SNF QRP
We proposed to require SNFs to collect and submit four new items as standardized patient assessment data elements under the SDOH category using the MDS: one item for Living Situation, as described in section VI.C.3.(a) of the proposed rule; two items for Food, as described in section VI.C.3.(b) of the proposed rule; and one item for Utilities, as described in section VI.C.3.(c) of the proposed rule.
We selected the SDOH items from the Accountable Health Communities (AHC) Health-Related Social Needs (HRSN) Screening Tool developed for the AHC Model.[21] The AHC HRSN Screening Tool is a universal, comprehensive screening for HRSNs that addresses five core domains as follows: (1) housing instability (for example, homelessness, poor housing quality); (2) food insecurity; (3) transportation difficulties; (4) utility assistance needs; and (5) interpersonal safety concerns (for example, intimate-partner violence, elder abuse, child maltreatment).[22]
We believe that requiring SNFs to report the Living Situation, Food, Utilities, and Transportation items that are included in the AHC HRSN Screening Tool will further standardize the screening of SDOH across quality programs. For example, as outlined in the proposed rule, our proposal will align, in part, with the requirements of the Hospital Inpatient Quality Reporting (IQR) Program and the Inpatient Psychiatric Facility Quality Reporting (IPFQR) Program. As of January 2024, hospitals are required to report whether they have screened patients for the standardized SDOH categories of housing instability, food insecurity, utility difficulties, transportation needs, and interpersonal safety to meet the Hospital IQR Program requirements.[23] Additionally, beginning January 2025, IPFs will also be required to report whether they have screened patients for the same set of SDOH categories.[24] As we continue to standardize data collection across PAC settings, we believe using common standards and definitions for new items is important to promote interoperable exchange of longitudinal information between SNFs and other providers to facilitate coordinated care, continuity in care planning, and the discharge planning process.
Below we describe each of the four items in more detail.
(a) Living Situation
Healthy People 2030 prioritizes economic stability as a key SDOH, of which housing stability is a component.[25 26] Lack of housing stability encompasses several challenges, such as having trouble paying rent, overcrowding, moving frequently, or spending the bulk of household income on housing.[27] These experiences may negatively affect one's physical health and access to health care. Housing instability can also lead to homelessness, which is housing deprivation in its most severe form.[28] On a single night in 2023, roughly 653,100 people, or 20 out of every 10,000 people in the United States, were experiencing homelessness.[29] Studies also found that people who are homeless have an increased risk of premature death and experience chronic disease more often than among the general population.[30] We believe that SNFs can use information obtained from the Living Situation item during a resident's discharge planning. For example, SNFs could work in partnership with community care hubs and community-based organizations to establish new care transition workflows, including referral pathways, contracting mechanisms, data sharing strategies, and implementation training that can track HRSNs to ensure unmet needs, such as housing, are successfully addressed through closed loop referrals and follow-up.[31] SNFs could also take action to help alleviate a resident's other related costs of living, like food, by referring the resident to community-based organizations that would allow the resident's additional resources to be allocated towards housing without sacrificing other needs.[32] Finally, SNFs could use the information obtained from the Living Situation item to better coordinate with other healthcare providers, facilities, and agencies during transitions of care, so that referrals to address a resident's housing stability are not lost during vulnerable transition periods.
Due to the potential negative impacts housing instability can have on a resident's health, we proposed to adopt the Living Situation item as a new standardized patient assessment data element under the SDOH category. The proposed Living Situation item is based on the Living Situation item collected in the AHC HRSN Screening Tool,[33 34] and was adapted from the Protocol for Responding to and Assessing Patients' Assets, Risks, and Experiences (PRAPARE) tool.[35] The proposed Living Situation item asks, “What is your living situation today?” The proposed response options are: (0) I have a steady place to live; (1) I have a place to live today, but I am worried about losing it in the future; (2) I do not have a steady place to live; (7) Resident declines to respond; and (8) Resident unable to respond. A draft of the Living Situation item proposed as a standardized patient assessment data element under the SDOH category can be found in the Downloads section of the SNF QRP Measures and Technical Information web page at https://www.cms.gov/medicare/quality/snf-quality-reporting-program/measures-and-technical-information.
(b) Food
The U.S. Department of Agriculture, Economic Research Service defines a lack of food security as a household-level economic and social condition of limited or uncertain access to adequate food.[36] Adults who are food insecure may be at an increased risk for a variety of negative health outcomes and health disparities. For example, a study found that food-insecure adults may be at an increased risk for obesity.[37] Another study found that food-insecure adults have a significantly higher probability of death from any cause or cardiovascular disease in long-term follow-up care, in comparison to adults that are food secure.[38]
While having enough food is one of many predictors for health outcomes, a diet low in nutritious foods is also a factor.[39] The United States Department of Agriculture (USDA) defines nutrition security as “consistent and equitable access to healthy, safe, affordable foods essential to optimal health and well-being.” [36] Nutrition security builds on and complements long standing efforts to advance food security. Studies have shown that older adults struggling with food insecurity consume fewer calories and nutrients and have lower overall dietary quality than those who are food secure, which can put them at nutritional risk.[40] Older adults are also at a higher risk of developing malnutrition, which is considered a state of deficit, excess, or imbalance in protein, energy, or other nutrients that adversely impacts an individual's own body form, function, and clinical outcomes.[41] Up to 50 percent of older adults are affected by or at risk for malnutrition, which is further aggravated by a lack of food security and poverty.[42] These facts highlight why the Biden-Harris Administration launched the White House Challenge to End Hunger and Build Health Communities.[43]
We believe that adopting items to collect and analyze information about a resident's food security at home could provide additional insight to their health complexity and help facilitate coordination with other healthcare providers, facilities, and agencies during transitions of care, so that referrals to address a resident's food security are not lost during vulnerable transition periods. For example, a SNF's dietitian or other clinically qualified nutrition professional could work with the resident and their caregiver to plan healthy, affordable food choices prior to discharge.[44] SNFs could also refer a resident that indicates lack of food security to government initiatives such as the Supplemental Nutrition Assistance Program (SNAP) and food pharmacies (programs to increase access to healthful foods by making them affordable), two initiatives that have been associated with lower health care costs and reduced hospitalization and emergency department visits.[45]
We proposed to adopt two Food items as new standardized patient assessment data elements under the SDOH category. These proposed items are based on the Food items collected in the AHC HRSN Screening Tool and were adapted from the USDA 18-item Household Food Security Survey (HFSS).[46] The first proposed Food item states, “Within the past 12 months, you worried that your food would run out before you got money to buy more.” The second proposed Food item states, “Within the past 12 months, the food you bought just didn't last and you didn't have money to get more.” We proposed the same response options for both items: (0) Often true; (1) Sometimes true; (2) Never True; (7) Resident declines to respond; and (8) Resident unable to respond. A draft of the Food items proposed to be adopted as standardized patient assessment data elements under the SDOH category can be found in the Downloads section of the SNF QRP Measures and Technical Information web page at https://www.cms.gov/medicare/quality/snf-quality-reporting-program/measures-and-technical-information.
(c) Utilities
A lack of energy (utility) security can be defined as an inability to adequately meet basic household energy needs.[47] According to the United States Department of Energy, one in three households in the U.S. are unable to adequately meet basic household energy needs.[48] The consequences associated with a lack of utility security are represented by three primary dimensions: economic; physical; and behavioral. Residents with low incomes are disproportionately affected by high energy costs, and they may be forced to prioritize paying for housing and food over utilities.[49] Some residents may face limited housing options, and therefore, are at increased risk of living in lower-quality physical conditions with malfunctioning heating and cooling systems, poor lighting, and outdated plumbing and electrical systems.[50] Residents with a lack of utility security may use negative behavioral approaches to cope, such as using stoves and space heaters for heat.[51] In addition, data from the Department of Energy's U.S. Energy Information Administration confirm that a lack of energy security disproportionately affects certain populations, such as low-income and African American households.[52] The effects of a lack of utility security include vulnerability to environmental exposures such as dampness, mold, and thermal discomfort in the home, which have a direct impact on a person's health.[53] For example, research has shown associations between a lack of energy security and respiratory conditions as well as mental health-related disparities and poor sleep quality in vulnerable populations such as the elderly, children, the socioeconomically disadvantaged, and the medically vulnerable.[54]
We believe adopting an item to collect information about a resident's utility security would facilitate the identification of residents who may not have utility security and who may benefit from engagement efforts. For example, SNFs may be able to use the information on utility security to help connect some residents in need to programs that can help older adults pay for their home energy (heating/cooling) costs, like the Low-Income Home Energy Assistance Program (LIHEAP).[55] SNFs may also be able to partner with community care hubs and community-based organizations to assist the resident in applying for these and other local utility assistance programs, as well as helping them navigate the enrollment process.[56]
We proposed to adopt a new item, Utilities, as a new standardized patient assessment data element under the SDOH category. This proposed item is based on the Utilities item collected in the AHC HRSN Screening Tool, and was adapted from the Children's Sentinel Nutrition Assessment Program (C-SNAP) survey.[57] The proposed Utilities item asks, “In the past 12 months, has the electric, gas, oil, or water company threatened to shut off services in your home?” The proposed response options are: (0) Yes; (1) No; (2) Already shut off; (7) Resident declines to respond; and (8) Resident unable to respond. A draft of the Utilities item proposed as a standardized patient assessment data element under the SDOH category can be found in the Downloads section of the SNF QRP Measures and Technical Information web page at https://www.cms.gov/medicare/quality/snf-quality-reporting-program/measures-and-technical-information.
4. Interested Parties Input
We developed our updates to add these items after considering feedback we received in response to our request for information (RFI) on “Principles for Selecting and Prioritizing SNF QRP Quality Measures and Concepts Under Consideration for Future Years” in the FY 2024 SNF PPS final rule (88 FR 53265 through 53267). This RFI sought to obtain input on a set of principles to identify SNF QRP measures, as well as additional thoughts about measurement gaps, and suitable measures for filling these gaps. In response to this solicitation, many commenters generally stated that the inclusion of a malnutrition screening and intervention measures would promote both quality and health equity. Other measures and measurement concepts included health equity, psychosocial issues, and caregiver status. The FY 2024 SNF PPS final rule includes a summary of the public comments that we received in response to the RFI and our responses to those comments (88 FR 53265 through 53267).
We also considered comments received in response to our Health Equity Update in the FY 2024 SNF PPS final rule. Comments were generally supportive of CMS' efforts to develop ways to measure and mitigate health inequities. One commenter referenced their belief that collection of SDOH would enhance holistic care, call attention to impairments that might be mitigated or resolved, and facilitate clear communication between residents and SNFs. While there were commenters who urged CMS to balance reporting requirements so as not to create undue administrative burden, another commenter suggested CMS incentivize collection of data on SDOH such as housing stability and food security. The FY 2024 SNF PPS final rule (88 FR 53268 through 53269) includes a summary of the public comments that we received in response to the Health Equity Update and our responses to those comments.
Additionally, we considered feedback we received when we proposed the creation of the SDOH category of standardized patient assessment data elements in the FY 2020 SNF PPS proposed rule (84 FR 17671 through 17679). Commenters were generally in favor of the concept of collecting SDOH items and stated that, if implemented appropriately, the data could be useful in identifying and addressing health care disparities, as well as refining the risk adjustment of outcome measures. The FY 2020 SNF PPS final rule (84 FR 38805 through 38818) includes a summary of the public comments that we received and our responses to those comments. We incorporated this input into the development of this update.
We solicited comment on the proposal to adopt four new items as standardized patient assessment data elements under the SDOH category beginning with the FY 2027 SNF QRP: one Living Situation item; two Food items; and one Utilities item.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: Many commenters supported the proposed new SDOH assessment items, viewing this as an important step towards identifying health disparities, improving health outcomes, understanding diverse resident needs, improving discharge planning and care coordination, and fostering continuous quality improvement. Many of these commenters also emphasized the importance of SDOH data collection in achieving health equity, and one commenter emphasized the importance of identifying, documenting, and addressing SDOH to provide equitable, high-quality, holistic, resident-centered care. Several commenters noted the importance of the proposed new SDOH assessment items in facilitating discharge planning strategies that can account for a person's housing, food, utilities, and transportation needs. One of these commenters agreed that risk factors such as a person's living situation in the community, and access to adequate nutrition and utilities necessary for a safe and health-promoting environment, need to be identified and addressed in the plan of care. This commenter went on to say that reducing housing, food, utility, and transportation security barriers as part of a SNF's discharge planning processes can reduce the risk for negative outcomes, such as hospital readmissions and readmission to the nursing facility for long-term care, when they return to the community. One of these commenters noted that collecting more granular SDOH data is crucial, especially for those residents who transition from SNFs to home or community-based settings. Two of these commenters also noted that the lack of information on residents' social risk factors is a barrier to providing social services to high-risk and underserved populations and believe the value of including data collection on these new assessment items outweighs the additional administrative burden.
Response: We appreciate the support. We agree that the collection of the new SDOH assessment items will support SNFs that wish to understand the health disparities that affect their resident populations, facilitate coordinated care, foster continuity in care planning, and assist with the discharge planning process from the SNF setting.
Comment: One commenter supported CMS's decision to align and standardize new SDOH data collection in the SNF QRP with data already being collected in other settings, such as the Hospital Inpatient Quality Reporting (IQR) Program and the Inpatient Psychiatric Facility Quality Reporting (IPFQR) Program requirements.
Response: We thank the commenter for recognizing that our proposal aligns, in part, with the requirements of the Hospital IQR Program and the IPFQR Program. as we continue to standardize data collection across settings, we believe using common standards and definitions for new assessment items is important to promote interoperable exchange of longitudinal information between SNFs and other providers. We also believe collecting this information may facilitate coordinated care, continuity in care planning, and the discharge planning process from PAC settings, including SNFs.
Comment: Several commenters agreed with the importance of collecting SDOH assessment items through the MDS, but also expressed concerns about the additional administrative burden associated with collecting the proposed SDOH data beginning in FY 2025 for the FY 2027 SNF QRP. Several of these commenters noted that data collection is financially burdensome and increases burden on already overextended staff. One commenter noted that because CMS proposed to add the assessment items to the MDS, SNFs would also be required to collect this data on Medicaid residents as well, which would add to the reporting and administrative burden. Another commenter requested additional funding for the increased costs associated with what they noted to be tasks outside the normal day-to-day operations of the facilities.
Response: Although the addition of four new SDOH assessment items to the MDS will increase the burden associated with completing the MDS, we carefully considered this increased burden against the benefits of adopting the assessment items for the MDS. Collection of additional SDOH assessment items will permit us to continue developing the statistical tools necessary to maximize the value of Medicare data and improve the quality of care for all beneficiaries, and therefore we do not want to delay the implementation of the new SDOH assessment items. As noted in section VI.C.2 of the proposed rule (89 FR 23464) and section VII.C.2 of this final rule, we recently developed and released the Health Equity Confidential Feedback Reports, which provided data to SNFs on whether differences in quality measure outcomes are present for their residents by dual-enrollment status and race and ethnicity.[58] In balancing the reporting burden for SNFs, we prioritized our policy objective to collect additional SDOH standardized patient assessment data elements that will inform care planning and coordination and quality improvement across care settings.
Regarding the comment requesting additional funding for the increased costs associated with collecting data on these new assessment items, we find the comment unclear. We interpret the commenter to mean that they do not believe that current SNF PPS payments are sufficient to cover the increased burden (specifically, costs) associated with collection of this additional data for the proposed new SDOH assessment items. As discussed previously, we carefully considered the increased burden associated with collection of these four new SDOH assessment items against the benefits of adopting these items for the MDS. This collection could be useful to SNFs as they identify the discharge needs of each resident. This includes developing and implementing an effective discharge planning process that focuses on the resident's discharge goals, preparing residents to be active partners, effectively transitioning them to post-discharge care, and reducing factors leading to preventable readmissions. The new SDOH assessment items we proposed to adopt were identified in the 2016 NASEM report [59] or the 2020 NASEM report [60] as impacting care use, cost, and outcomes for Medicare beneficiaries. We believe the proposed new SDOH assessment items have the potential to generate actionable data SNFs can use to implement effective discharge planning processes that can reduce the risk for negative outcomes such as hospital readmissions and admission to a nursing facility for long-term care. Given that SNFs must develop and implement an effective discharge planning process that ensures the discharge needs of each resident are identified, we believe SNFs are likely collecting some of this data already. Collection of these new SDOH items will provide key information to SNFs to support effective discharge planning.
Regarding the commenter's concern that SNFs would be required to collect this data on Medicaid residents, it is unclear specifically what the commenter's concerns are. In section VII.E.3. of this final rule, we proposed to adopt four new SDOH assessment items for the SNF QRP. For the SNF QRP, SNFs are required to collect and submit data for MDS items specified by CMS for Medicare Part A fee-for service residents receiving skilled services. We did not propose and would not require SNFs to collect and submit data for the four new SDOH assessment items and modified Transportation item on Medicaid residents residing in the nursing facility.
Finally, we plan to provide training resources in advance of the initial collection of the new SDOH assessment items to ensure that SNFs have the tools necessary to administer these new items and reduce the burden to SNFs having to create their own training resources. These training resources may include online learning modules, tip sheets, questions and answers documents and/or recorded webinars and videos. We anticipate that we will make these materials available to SNFs in mid-2025, which will give SNFs several months prior to required collection and reporting to take advantage of the learning opportunities.
Comment: One commenter who supported the proposal to collect the new and modified SDOH assessment items, also encouraged CMS to ensure the new assessment items are valid and reliable. Two commenters, who did not support the proposal, noted concerns with the validity and reliability of the proposed new and modified SDOH assessment items, and one of these commenters recommended further testing of the proposed items.
Response: We disagree that the proposed new SDOH assessment items require further testing prior to requiring SNFs to collect them on the MDS for the SNF QRP. The AHC HRSN Screening Tool is evidence-based and informed by practical experience. With input from a panel of national experts convened by our contractor, We developed the tool under the Center for Medicare and Medicaid Innovation (CMMI) by conducting a review of existing screening tools and questions focused on core and supplemental HRSN domains, including housing instability, food insecurity, transportation difficulties, utility assistance needs, and interpersonal safety concerns.[61] These domains were chosen based upon literature review and expert consensus utilizing the following three criteria: (1) availability of high-quality scientific evidence linking a given HRSN to adverse health outcomes and increased healthcare utilization, including hospitalizations and associated costs; (2) ability for a given HRSN to be screened and identified in the inpatient setting prior to discharge, addressed by community-based services, and potentially improve healthcare outcomes, including reduced readmissions; and (3) evidence that a given HRSN is not systematically addressed by healthcare providers.[62] In addition to established evidence of their association with health status, risk, and outcomes, these domains were selected because they can be assessed across the broadest spectrum of individuals in a variety of settings.[63 64]
Through this process, over 50 screening tools totaling more than 200 questions were compiled. To refine this list, CMS' contractor consulted a technical expert panel (TEP) consisting of a diverse group of tool developers, public health and clinical researchers, clinicians, population health and health systems executives, community-based organization leaders, and Federal partners. Over the course of several meetings, this TEP met to discuss opportunities and challenges involved in screening for HRSNs; consider and pare down CMS's list of evidence-based screening questions; and recommend a short list of questions for inclusion in the final tool. The AHC HRSN Screening Tool was tested across many care delivery sites in diverse geographic locations across the United States. More than one million Medicare and Medicaid beneficiaries have been screened using the AHC HRSN Screening Tool. This tool was evaluated psychometrically and demonstrated evidence of both reliability and validity, including inter-rater reliability and concurrent and predictive validity. Moreover, the AHC HRSN Screening Tool can be implemented in a variety of places where individuals seek healthcare, including SNFs.
We selected these proposed assessment items for the SNF QRP from the AHC HRSN Screening Tool because we believe that collecting information on living situation, food, utilities, and transportation could have a direct and positive impact on resident care in SNFs. Specifically, collecting this information provides an opportunity for the SNF to identify residents' potential HRSNs, and if indicated, to address those with the resident, their caregivers, and community partners during the discharge planning process, potentially resulting in improvements in resident outcomes.
Comment: One commenter referenced CMS' second evaluation of the AHC model from 2018 through 2021,[65] and said they interpret the Findings at a Glance to conclude the AHC HRSN Screening Tool “did not appear to increase beneficiaries' connection to community services or HRSN resolution.”
Response: This two-page summary of the AHC Model 2018-2021 [66] describes the results of testing whether systematically identifying and connecting beneficiaries to community resources for their HRSNs improved health care utilization outcomes and reduced costs. To ensure consistency in the screening offered to beneficiaries across both an individual community's clinical delivery sites and across all the communities in the model, we developed a standardized HRSN screening tool. This AHC HRSN Screening Tool was used to screen Medicare and Medicaid beneficiaries for core HRSNs to determine their eligibility for inclusion in the AHC Model. If a Medicare or Medicaid beneficiary was eligible for the AHC Model, they were randomly assigned to one of two tracks: (1) Assistance; or (2) Alignment. The Assistance Track tested whether navigation assistance that connects navigation-eligible beneficiaries with community services results in increased HRSN resolution, reduced health care expenditures, and unnecessary utilization. The Alignment Track tested whether navigation assistance, combined with engaging key interested parties in continuous quality improvement (CQI) to align community service capacity with beneficiaries' HRSNs, results in greater increases in HRSN resolution and greater reductions in health expenditures and utilization than navigation assistance alone. Regardless of assigned track, all beneficiaries received HRSN screening, community referrals, and navigation to community services.[67]
We believe the commenter inadvertently misinterpreted the findings, believing these findings were with respect to the effectiveness and scientific validity of the AHC HRSN Screening Tool itself. The findings section of this two-page summary described six key findings from the AHC Model, which examined whether the Assistance Track or the Alignment Track resulted in greater increases in HRSN resolution and greater reductions in health expenditures and utilization. Particularly, the AHC Model reduced emergency department visits among Medicaid and FFS Medicare beneficiaries in the Assistance Track, which was suggestive that navigation may help patients use the health care system more effectively. We acknowledge that navigation alone did not increase beneficiaries' connection to community services or HRSN resolution, and this was attributed to gaps between community resource availability and beneficiary needs. The AHC HRSN Screening Tool used in the AHC Model was limited to identifying Medicare and Medicaid beneficiaries with at least one core HRSN who could be eligible to participate in the AHC Model. Our review of the AHC Model did not identify any issues with the validity and scientific reliability of the AHC HRSN Screening Tool.
Finally, as part of our routine item and measure monitoring work, we continually assess the implementation of new assessment items, and we will include the four new proposed SDOH assessment items in our monitoring work.
Comment: Two commenters requested that CMS articulate its vision for how the data collected from the proposed SDOH standardized patient assessment data elements will be used in quality and payment programs. These commenters were concerned that CMS may use the SDOH assessment data to develop a SNF QRP measure that would hold SNFs solely accountable for social drivers of health that require resources and engagement across an entire community to address. One of these commenters recommended that CMS not finalize this proposal and instead engage interested parties in the industry to understand the role that SNFs can play in improving SDOH.
Response: We proposed the four new SDOH assessment items because collection of additional SDOH items would permit us to continue developing the statistical tools necessary to maximize the value of Medicare data and improve the quality of care for all beneficiaries. For example, we recently developed and released the Health Equity Confidential Feedback Reports, which provided data to SNFs on whether differences in quality measure outcomes are present for their residents by dual-enrollment status and race and ethnicity.[68] We note that advancing health equity by addressing the health disparities that underlie the country's health system is one of our strategic pillars [69] and a Biden-Harris Administration priority.[70] Furthermore, any updates to the SNF QRP measure set would be addressed through future notice-and-comment rulemaking, as necessary.
Comment: One commenter said they recognize the importance of collecting standardized patient assessment data elements to better serve residents' needs and for identifying and addressing potential issues of equity. However, they urged CMS to reevaluate the utility of collecting this information, particularly compared to the burden of data collection. Specifically, they noted that CMS must keep the role of the social worker in a SNF in mind when considering these assessment items. They stated that a social worker's job in a SNF is to meet the needs of SNF residents during their SNF stay and to coordinate services for a successful return to the community, but the SNF social worker has no control over what happens after the resident discharges from the SNF and cannot become the resident's community social worker. Therefore, they believe a SNF's responses to the proposed new and modified SDOH assessment items would neither impact nor be impacted by the SNF stay.
Response: While we recognize the role that social workers have in the SNF, we believe that the proposed new and modified SDOH assessment items are relevant to the SNF's interdisciplinary care team and could impact the discharge planning occurring during the SNF stay. We proposed the collection of new and modified SDOH assessment items at the time of admission to the SNF because we believe that having information on residents' living situation, food, and utilities will give SNFs an opportunity to better understand and address the broader needs of their residents. We also believe this information is essential for comprehensive resident care, potentially leading to improved health outcomes and more effective discharge planning. As we stated in the proposed rule and in section VII.C.2 of this final rule, according to the World Health Organization, research shows that SDOH can be more important than health care or lifestyle choices in influencing health, accounting for between 30 to 55 percent of health outcomes.[71] This is part of a growing body of research that highlights the importance of SDOH on health outcomes. As noted previously, SNFs are already required by our regulation at § 483.21(c)(1) to develop and implement an effective discharge planning process.
Comment: One of these commenters did not agree with CMS that the proposed SDOH assessment items would produce interoperable data within the CMS quality programs because the proposed requirements for SNF are not standardized with the SDOH collection requirements in the Hospital IQR Program and IPFQR Programs. This commenter noted that the Screening for SDOH measures in the Hospital IQR and IPFQR Programs do not specify when a patient is screened (for example, at admission) and how the screening questions are asked (in other words, specific wording and responses). Instead, providers reporting these measures under the Hospital IQR and IPFQR Programs are only asked to document that a patient was screened for the following domains: housing instability, food insecurity, transportation difficulties, utility assistance needs, and interpersonal safety concerns.
Response: We disagree that the proposed collection of four new SDOH assessment items and one modified SDOH assessment item for the SNF QRP and the requirements for the Hospital IQR and IPFQR Programs do not promote standardization. Although hospitals and IPFs participating in these programs can use a self-selected SDOH screening tool, the Screening for SDOH and Screen Positive Rate for SDOH measures we have adopted for the Hospital IQR and IPFQR Programs address the same SDOH domains that we have proposed to collect as standardized patient assessment data under the SNF QRP: housing instability, food insecurity, utility difficulties, and transportation needs. We believe that this partial alignment will facilitate longitudinal data collection on the same topics across healthcare settings. As we continue to standardize data collection, we believe using common standards and definitions for new assessment items is important to promote the interoperable exchange of longitudinal information between SNFs and other providers to facilitate coordinated care, continuity in care planning, and the discharge planning process. This is evidenced by our recent proposals to add these four SDOH assessment items and one modified SDOH assessment item in the IRF QRP (89 FR 22275 through 22280), LTCH QRP (89 FR 36345 through 36350), and Home Health QRP (89 FR 55383 through 55388).
(a) Comments on the Living Situation Assessment Item
Comment: Several commenters supported the proposal to adopt the Living Situation item as a standardized patient assessment data element in the MDS. Several of these commenters emphasized that having information on living situation is critical for developing tailored and effective discharge plans. Two of these commenters noted that this information will allow providers to better understand social and environmental factors that affect their residents' health outcomes, and one of these commenters also noted that collecting and reporting living situation data could encourage SNFs to care for residents who may have more difficult discharges. Another commenter noted that having living situation information enables better care coordination, identifies support gaps, and allows SNFs to develop tailored care plans. Finally, another commenter noted that understanding a person's living situation can ensure the appropriate provision of necessary adaptive equipment to address their needs.
Response: We agree that a person's living situation may negatively affect their physical health and access to health care, and that SNFs can use information obtained from the Living Situation item for discharge planning, partnerships with community care hubs and community-based organizations, and coordination with other healthcare providers, facilities, and agencies during transitions of care.
Comment: One commenter recommended that the Living Situation item incorporate information on whether a resident's living situation is suitable for their potentially new complex care needs. This commenter highlighted the changing nature of SNF residents' needs and noted that some residents may have been housing secure prior to their condition, but their prior living situation may no longer be suitable for their current needs, which may include specific requirements such as mobility equipment.
Response: While we proposed to require the collection of the Living Situation item at admission only, the collection could potentially prompt the SNF to initiate additional conversations with their residents about their living situation needs throughout their stay. As the commenter pointed out, it is important to think about the resident's living situation in the context of their new care needs, and collecting the Living Situation assessment item at admission would be an important first step to that process. Additionally, SNFs may seek to collect any additional information that they believe may be relevant to their resident population to inform their care and discharge planning process.
Comment: One commenter recommended that a timeframe be added to the response options for the proposed Living Situation item. This commenter suggested that adding a timeframe of one year or less to these response options would allow healthcare providers to promptly intervene and mitigate any eminent negative housing situations. They were concerned that, if left open-ended, residents may respond yes, thinking about many possible scenarios that may occur in the distant future.
Response: We interpret the comment to be suggesting that a time frame be added to two of the Living Situation response options, specifically: (1) I have a place to live today, but I am worried about losing it in the future; and (2) I do not have a steady place to live. We want to clarify that the proposed Living Situation item frames the question as, “What is your living situation today?” The question establishes the timeframe (the present) the resident should consider in responding to the item.
Comment: Two commenters recommended that instead of collecting data on the proposed Living Situation assessment item, CMS should propose an item to collect information on financial insecurity. Both commenters stressed that financial insecurity underpins all the proposed SDOH items. One of these commenters encouraged CMS to eventually develop a mechanism to ensure that such needs are not only assessed but met with delivered services.
Response: We will consider this feedback as we evaluate future policy options. We note that although we proposed to require the collection of the Living Situation item for the SNF QRP, nothing would preclude SNFs from choosing to screen their residents for additional SDOH they believe are relevant for their resident population and the community they serve, including financial insecurity.
(b) Comments on the Food Assessment Items
Comment: We received several comments supporting the collection of the two proposed Food assessment items because of the importance of nutrition and food access to SNF residents' health outcomes, and the usefulness of this information for treatment and discharge planning. Specifically, two of these commenters highlighted the association between food insecurity and malnutrition with health outcomes, and one of these commenters highlighted the importance of addressing food insecurity among Medicare residents, particularly among elderly residents or those with chronic conditions. This commenter noted that addressing food security will help foster better health outcomes, lower healthcare costs, and enhance quality of life. Another one of these commenters noted that the responses to the Food assessment items would help providers incorporate treatment strategies that address residents' food access and guide the selection of interventions and training (for example, meal planning) provided throughout the plan of care. Moreover, another one of these commenters noted that the two proposed Food assessment items are critical to facilitating coordination with other healthcare providers and community-based organizations during transitions of care for residents at risk for inadequate food intake or who may need support in accessing healthy foods aligned with medically tailored meals or prescription diets. Finally, another commenter acknowledged the intersection between these proposed SDOH assessment items, highlighting the important relationship between transportation and a person's ability to access food. This commenter provided the example that a person may have enough funds to purchase food, but not have access to transportation to obtain food.
Response: We agree that a person's access to food affects their health outcomes and risk for adverse events, and understanding the potential needs of residents admitted to a SNF through the collection of the two new Food assessment items can help SNFs facilitate resources to better address a SNF resident's access to food when discharged.
Comment: One commenter did not support the proposed Food assessment items stating that, although the assessment items are valid, they do not provide clear information on nutritional status because there could be family members or community organizations that provide food support. Additionally, this commenter noted that “food” is a general term and does not address selection or intake of food.
Response: While we acknowledge that the proposed Food assessment items do not ask for specific information on residents' nutritional status or whether they have family members or community organizations that provide food support, our intent was to collect information on whether the resident may have worries about their access to food or are experiencing concerns about access to food. We believe that adopting the proposed Food assessment items will help SNFs identify any potential issues. Having this information could also help SNFs coordinate care upon discharge of their residents. We also note that, while the proposal would require the collection of the Food assessment items at admission only, the collection could potentially prompt the SNF to initiate conversations between the SNF and its residents about their food needs throughout their stay. Finally, we remind the commenter that nothing would preclude the SNF from choosing to screen its residents for additional SDOH they believe are relevant for their resident population and the community they serve, including family or community support.
Comment: One commenter expressed concerns that the proposed Food assessment items ask residents to rate the frequency of food shortages using a three-point scale, which is inconsistent with other questions on the MDS such as the resident mood, behavioral symptoms, and daily preference assessment items, which use a four-point scale to determine frequency. This commenter noted that this inconsistency may lead to confusion for staff and residents.
Response: We clarify that the proposed draft Food assessment items include three frequency responses in addition to response options in the event the resident declines to respond or is unable to respond: (0) Often true; (1) Sometimes true; (2) Never True; (7) Resident declines to respond; and (8) Resident unable to respond. We acknowledge that there are a number of resident interview assessment items on the MDS that use a four-point scale, but there are also assessment items on the MDS that do not use a four-point scale. For example, the Health Literacy (B1300), Social Isolation (D0700), and the Pain Interference with Therapy Activities (J0520) assessment items currently use a five-point scale item. We chose the proposed Food assessment items from the AHC HRSN Screening Tool, and they were tested and validated using a three-point response scale. Since the MDS currently includes assessment items that use varying response scales, we do not believe staff and residents will be confused. we plan to develop resources SNF staff can use to ensure residents understand the proposed item questions and response options. For example, we developed cue cards to assist SNFs in conducting the Brief Interview for Mental Status (BIMS) in Writing, the Resident Mood Interview (PHQ-2 to 9), the Pain Assessment Interview, and the Interview for Daily and Activity Preference.[72]
Comment: One commenter expressed concerns with the lack of evidence supporting the proposed Food assessment items in the older adult population and requested that CMS provide more detailed supporting evidence, or not finalize the proposal until it can produce such evidence. This commenter noted that the proposed Food assessment items were based on a research study for families with young children, and that they did not see information that would support their use in the older population.
Response: We interpret the commenter to be referring to the citation in the draft of the Food items posted on the SNF QRP Measures and Technical Information web page at https://www.cms.gov/medicare/quality/snf-quality-reporting-program/measures-and-technical-information. We acknowledge that the AHC Screening Tool includes a citation to a study that was done in children. However, as discussed in section VI.C.3(b) of the proposed rule and section VII.C.3(b) of this final rule, these items are also found in the USDA 18-item Household Food Security Survey (HFSS). The HFSS has been extensively used with adults both in the U.S. and internationally. More information about its use and research over the last 25 years can be found on the USDA website at https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-u-s/history-background/.
Comment: Two commenters were concerned with the 12-month look-back period for the proposed Food assessment items, noting that this broad look-back period may capture needs that occurred in the past, but have been resolved. These commenters recommended a three-month look-back period instead, to capture true concerns that should inform the SNFs' care and discharge planning.
Response: We disagree that the 12-month look back period for the proposed Food assessment items is too long and that it will not result in reliable responses. We believe a 12-month look back period is more appropriate than a shorter, three-month look-back period because a person's Food situation may fluctuate over time. One study of Medicare Advantage beneficiaries found that approximately half of U.S. adults report one or more HRSNs over four quarters.[73] However, at the individual level, participants had substantial fluctuations: 47.4 percent of the participants fluctuated between 0 and 1 or more HRSNs over the four quarters, and 21.7 percent of participants fluctuated between one, two, three, or four or more HRSNs over the four quarters. The researchers noted that the dynamic nature of individual-level HRSNs requires consideration by healthcare providers screening for HRSNs.
To account for potentially changing Food needs over time, we believe it is important to use a longer look-back period to comprehensively capture any Food needs a SNF resident may have had, so that SNFs may consider them in their care and discharge planning.
Comment: Three commenters recognized the importance of collecting information on residents' food access through a streamlined data collection process, but recommended that CMS combine the two proposed Food assessment items into a singular comprehensive assessment item to enhance efficiency and reduce respondent burden, while still capturing the nuanced aspects of food insecurity crucial for care planning and recourse allocation. Two of these commenters also noted that beneficiaries may be uncomfortable sharing this sensitive personal information with facility staff and may be reluctant to respond to two nearly identical questions.
Response: We appreciate the commenters' recommendation to combine the two separate proposed Food assessment items into a single comprehensive assessment item to reduce respondent burden. However, past testing of the items found that the item sensitivity was higher when using both Food assessment items, as opposed to just one. Specifically, these analyses found that an affirmative response to just one of the questions provided a sensitivity of 93 percent or 82 percent, depending on the item, whereas collecting both of the proposed Food items, and evaluating whether there is an affirmative response to the first and/or second item yielded a sensitivity of 97 percent.[74] This means that only 3 percent of respondents who have food needs were likely to be misclassified. Therefore, we believe it is important to include both proposed Food assessment items.
In response to commenters who noted that beneficiaries may be uncomfortable sharing this sensitive personal information with facility staff, we acknowledge that the Food assessment items require the resident to be asked potentially sensitive questions. We recommend that SNFs ensure residents feel comfortable answering these questions and explain to residents that the information will be helpful to developing an individualized plan of care and discharge plan. Additionally, the proposed items include a response option, (7) Resident declines to respond, for residents who may decline to respond to the proposed Food assessment items. Information provided by residents in response to the proposed Food assessment items may be protected health information (PHI),[75] and SNFs are responsible for adopting reasonable safeguards to ensure that residents' information is not impermissibly disclosed contrary to applicable confidentiality, security, and privacy laws.
We plan to provide training resources in advance of the initial collection of the proposed new Food assessment items to ensure that SNFs have the tools necessary to administer the new proposed new Food assessment items and reduce the burden to SNFs in creating their own training resources. These training resources may include online learning modules, tip sheets, questions and answers documents, and/or recorded webinars and videos, and would be available to providers in mid-2025, allowing SNFs several months to ensure their staff take advantage of the learning opportunities.
(c) Comments on the Utilities Assessment Item
Comment: Several commenters supported the proposal to add a new Utility assessment item to the MDS and highlighted that a resident's access to utilities is crucial for maintaining a safe and healthy living environment. These commenters noted that understanding residents' utility needs will help SNFs in their discharge planning. One of these commenters noted that by assessing a resident's utility security, SNFs may be able to improve their access by referring them to programs like the Low-Income Home Energy Assistance Program (LIHEAP) [76] or other organizations that provide assistance to those with utility needs. Two commenters highlighted that SNF residents are often discharged with equipment requiring constant, consistent electricity (for example, supplemental oxygen, vents, continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), continuous ambulatory delivery device (CADD) pumps for Dobutamine, and left ventricular assist device (LVAD). If a resident does not have access to a reliable power source for these critical supports, they are at risk of not using the equipment as prescribed or dying.
Response: We thank the commenters for their support and agree that residents' utilities needs can affect SNF residents' health outcomes, and the collection of the proposed Utilities assessment item can equip SNFs with the information to inform care plans and discharge planning.
Comment: Two commenters were concerned with the 12-month look-back period for the proposed Utility assessment item, noting that this broad look-back period may not result in reliable responses, or their needs may have been resolved. One of these commenters recommended a three-month look-back period instead, to provide more reliable, valid, timely, and actionable information for the transition of care.
Response: We disagree that the 12-month look back period for the proposed Utility assessment item is too long and that it will not result in reliable responses. We believe a 12-month look-back period is more appropriate than a shorter, 3-month look-back period because a person's Utilities situation may fluctuate over time. As we noted in an earlier response, a study of Medicare Advantage beneficiaries found that approximately half of U.S. adults report one or more HRSNs over 4 quarters. However, at the individual level, participants had substantial fluctuations: 47.4 percent of the participants fluctuated between 0 and 1 or more HRSNs over the four quarters, and 21.7 percent of participants fluctuated between one, two, three, or four or more HRSNs over the 4 quarters.[77] The researchers noted that the dynamic nature of individual-level HRSNs requires consideration by healthcare providers screening for HRSNs.
To account for potentially changing Utilities needs over time, we believe it is important to use a longer look-back period to comprehensively capture any Utilities needs a SNF resident may have had, so that SNFs may consider them in their care and discharge planning.
Comment: Two commenters suggested that CMS consider assessing family caregiver burden as well as services delivery, the latter of which would capture whether referrals to appropriate services resulted in actual service delivery. One of the commenters also recommended the inclusion of assessment items to improve the overall resident care among those with disabilities, such as: disability-status, residents' independent living status, and ability to return to work.
Response: We agree that it is important to understand family caregiver burden, service delivery, and the needs of residents with disabilities. as we continue to evaluate SDOH standardized patient assessment data elements and future policy options, we will consider this feedback. We note that although we proposed to require the collection of the Utilities item for the SNF QRP, nothing would preclude SNFs from choosing to screen their residents for additional SDOH they believe are relevant to their resident population and the community they serve, including screening for caregiver burden and service delivery.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt four new items as standardized patient assessment data elements under the SDOH category beginning with the FY 2027 SNF QRP: one Living Situation item; two Food items; and one Utilities item.
5. Modification of the Transportation Item Beginning With the FY 2027 SNF QRP
Beginning October 1, 2023, SNFs began collecting seven items adopted as standardized patient assessment data elements under the SDOH category on the MDS.[78] One of these items, Item A1250. Transportation, collects data on whether a lack of transportation has kept a resident from getting to and from medical appointments, meetings, work, or from getting things they need for daily living. This item was adopted as a standardized patient assessment data element under the SDOH category in the FY 2020 SNF PPS final rule (84 FR 38805 through 38809). As we stated in the FY 2020 SNF PPS final rule (84 FR 38814 through 42588), we continue to believe that access to transportation for ongoing health care and medication access needs, particularly for those with chronic diseases, is essential to successful chronic disease management and that the collection of a Transportation item would facilitate the connection to programs that can address identified needs (84 FR 38815 through 42588).
As part of our routine item and measure monitoring work, we continually assess the implementation of the new SDOH items. We have identified an opportunity to improve the data collection for A1250. Transportation in the MDS by aligning it with the Transportation category collected in our other programs.[79] Specifically, we proposed to modify the current Transportation item in the MDS so that it aligns with a Transportation item collected on the AHC HRSN Screening Tool, one of the potential tools the IPFQR and Hospital IQR Programs may select for data collection for the Screening for SDOH measure, as discussed previously.
A1250. Transportation collected in the MDS asks: “Has lack of transportation kept you from medical appointments, meetings, work, or from getting things needed for daily living?” The response options are: (A) Yes, it has kept me from medical appointments or from getting my medications; (B) Yes, it has kept me from non-medical meetings, appointments, work, or from getting things that I need; (C) No; (X) Resident unable to respond; and (Y) Resident declines to respond. The Transportation item collected in the AHC HRSN Screening Tool asks, “In the past 12 months, has lack of reliable transportation kept you from medical appointments, meetings, work or from getting things needed for daily living?” The two response options are: Yes; and No. Consistent with the AHC HRSN Screening Tool and adapted from the PRAPARE tool, we proposed to modify the A1250. Transportation item collected in the SNF MDS in two ways: (1) revise the look-back period for when the resident experienced lack of reliable transportation; and (2) simplify the response options.
First, the modification of the Transportation item would use a defined 12-month look back period, while the current Transportation item uses a look back period of 6 to 12 months. We believe the distinction of a 12-month look back period would reduce ambiguity for both residents and clinicians, and therefore, improve the validity of the data collected. Second, we proposed to simplify the response options. Currently, SNFs separately collect information on whether a lack of transportation has kept the patient from medical appointments or from getting medications, and whether a lack of transportation has kept the resident from non-medical meetings, appointments, work, or from getting things they need. Although transportation barriers can directly affect a person's ability to attend medical appointments and obtain medications, a lack of transportation can also affect a person's health in other ways, including accessing goods and services, obtaining adequate food and clothing, and social activities.[80] The modified Transportation item would collect information on whether a lack of reliable transportation has kept the resident from medical appointments, meetings, work or from getting things needed for daily living, rather than collecting the information separately. As discussed previously, we believe reliable transportation services are fundamental to a person's overall health, and as a result, the burden of collecting this information separately outweighs its potential benefit.
For the reasons outlined in the proposed rule, we proposed to modify A1250. Transportation based on the Transportation item adopted for use in the AHC HRSN Screening Tool and adapted from the PRAPARE tool. The Transportation item asks, “In the past 12 months, has a lack of reliable transportation kept you from medical appointments, meetings, work or from getting things needed for daily living?” The response options are: (0) Yes; (1) No; (7) Resident declines to respond; and (8) Resident unable to respond. A draft of the proposed modified Transportation item can be found in the Downloads section of the SNF QRP Measures and Technical Information web page at https://www.cms.gov/medicare/quality/snf-quality-reporting-program/measures-and-technical-information.
We solicited comment on the proposal to modify the current Transportation item previously adopted as a standardized patient assessment data element under the SDOH category beginning with the FY 2027 SNF QRP.
We received public comments on this proposal. The following is a summary of the comments we received and our responses.
Comment: Several commenters supported the proposal to modify the Transportation assessment item. Two commenters supported the simplified response options, noting that it would make it easier for residents to answer the question. These commenters also expressed support for the new 12-month look-back period because it would help clarify the question, improve resident comprehension of the proposed Transportation assessment item, and reduce provider burden. Another commenter noted that knowing this information will allow the SNF to connect residents, particularly those who are dependent on a wheelchair or other assisted device for mobility, with reliable transportation services.
Response: We thank the commenters for their support of the proposed modification of the Transportation assessment item. We agree that the proposed changes would help streamline the data collection process by simplifying the item for both residents and SNF staff that collect the data. The use of a 12-month look-back period will reduce ambiguity for both residents and staff, and therefore, improve the validity of the data collected.
Comment: Two commenters expressed concerns about the 12-month look-back period, noting that it may not offer reliable and valid information, and recommended a 3-month look-back period instead. Both commenters also noted that there are limitations with the response options because the responses do not allow for understanding the frequency of the concern, the reasons why reliable transportation is not available or the special accommodations a person may need for transportation.
Response: We disagree that the 12-month look-back period for the proposed modification to the Transportation assessment item is too long and that it will not result in reliable responses. We believe a 12-month look-back period is more appropriate than a shorter, three-month look-back period because a person's Transportation needs may fluctuate over time. As we have noted in an earlier response, a study of Medicare Advantage beneficiaries found that approximately half of U.S. adults report one or more HRSNs over 4 quarters. However, at the individual level, participants had substantial fluctuations: 47.4 percent of the participants fluctuated between 0 and 1 or more HRSNs over the 4 quarters, and 21.7 percent of participants fluctuated between one, two, three, or four or more HRSNs over the 4 quarters.[81] The researchers noted that the dynamic nature of individual-level HRSNs requires consideration by healthcare providers screening for HRSNs. To account for potentially changing Transportation needs over time, we believe it is important to use a longer look-back window to comprehensively capture any Transportation needs a person may have had, so that SNFs may consider them in their care and discharge planning.
Regarding the comment stating the responses do not allow for nuanced understanding of the resident's transportation needs (the frequency of the concern, the reasons why reliable transportation is not available, or the special accommodations a person may need for transportation), we note that although the proposal would require the collection of the Transportation assessment item at admission only, the collection could potentially prompt the SNF to initiate conversations with its residents about their specific Transportation needs. Additionally, SNFs may seek to collect any additional information that they believe may be relevant to their resident population to inform their care and discharge planning process.
After careful consideration of the public comments we received, we are finalizing our proposal to modify the current Transportation item previously adopted as a standardized patient assessment data element under the SDOH category beginning with the FY 2027 SNF QRP.
D. SNF QRP Quality Measure Concepts Under Consideration for Future Years—Request for Information (RFI)
In the proposed rule, we solicited input on the importance, relevance, appropriateness, and applicability of each of the concepts under consideration listed in Table 29 for future years in the SNF QRP. The FY 2024 SNF PPS proposed rule (88 FR 21353 through 21355) included a request for information (RFI) on a set of principles for selecting and prioritizing SNF QRP measures, identifying measurement gaps, and suitable measures for filling these gaps. We also sought input on data available to develop measures, approaches for data collection, perceived challenges or barriers, and approaches for addressing identified challenges. We refer readers to the FY 2024 SNF PPS final rule (88 FR 53265 through 53267) for a summary of the public comments we received in response to the RFI.
Subsequently, our measure development contractor convened a Technical Expert Panel (TEP) on December 15, 2023, to obtain expert input on the future measure concepts that could fill the measurement gaps identified in our FY 2024 RFI.[82] The TEP also discussed the alignment of PAC and Hospice measures with CMS' “Universal Foundation” of quality measures.[83]
In consideration of the feedback we have received through these activities, we solicited input on four concepts for the SNF QRP (See Table 29). One is a composite of vaccinations [84] which could represent overall immunization status of residents such as the Adult Immunization Status measure [85] in the Universal Foundation. A second concept on which we sought feedback is the concept of depression for the SNF QRP, which may be similar to the Clinical Screening for Depression and Follow-up measure [86] in the Universal Foundation. Finally, we sought feedback on the concepts of pain management and patient experience of care/patient satisfaction for the SNF QRP.
We received public comments on this RFI. The following is a summary of the comments we received.
1. Vaccination Composite
Comment: Most commenters stated they understand CMS' efforts to promote vaccination among residents, and many commenters supported the idea of adding a composite vaccination measure like the Adult Immunization Status (AIS) measure into the SNF QRP. One commenter noted that a composite vaccination measure could improve vaccination rates for those vaccines recommended by the Advisory Committee on Immunization Practices (ACIP), reduce administrative burden through alignment with the Universal Foundation,[87] and potentially improve immunization rates in PAC settings, including SNFs. Another commenter noted that vaccines may not only help prevent illness, or minimize symptoms, but also save lives, especially for key conditions including COVID-19, influenza, respiratory syncytial virus (RSV), and pneumonia that have the most severe impact on older adults and individuals with multiple chronic conditions that receive post-acute or long-term care in nursing homes. Another commenter noted that, while in previous years they have shared concerns on the Patient/Resident COVID-19 Vaccine measure in rulemaking comments, if this measure is rolled into a composite vaccination measure, they would support the concept, particularly if the weight of the COVID-19 vaccination for residents is weighed appropriately in relation to the influenza vaccine.
Several commenters, however, did not support the idea of adding a composite vaccination measure into the SNF QRP for a number of reasons. They questioned whether the SNF is the appropriate setting for collecting vaccination rates, and pointed to several challenges SNFs would experience in gathering information on vaccination status and insuring the validity of the measure.
Two commenters suggested that a composite vaccination measure should focus on primary care practices as the appropriate setting in which to report vaccination status, and this information could be shared with other healthcare providers when a resident requires services in another setting. Another commenter did not support the use of composite vaccination measures stating that they may mask specific vaccination uptake and make it more difficult to interpret vaccination status. This commenter recommended that CMS report on specific vaccination rates because it would provide more actionable data to SNFs. One of these commenters also questioned whether there would be exclusions for medical contraindications and deeply held religious beliefs, and how a measure reported by residents in the SNF would be verified.
Three commenters also noted that there are numerous reasons beyond health contraindications that residents may decide whether to receive vaccinations, and these reasons are largely dependent on factors outside of a SNF's control, such as where the facility is located and personal preference of the residents. Two of these commenters suggested that, by requiring a composite vaccination measure, a SNF could be incentivized either not to offer admission to residents who are not up to date with vaccinations or admit the resident and administer the vaccinations, even when vaccine administration may increase the risk of adverse health outcomes.
2. Pain Management
Comment: Most commenters supported the pain management measure concept. One of these commenters noted that a resident's experience of pain can affect numerous aspects of their care, including their ability to tolerate therapy, their ability to gain function, their mental health, and their overall experience of care. Another one of these commenters stated that these measures could potentially inform future efforts to address inequities in SNF care. Three of these commenters urged CMS to recognize the value of nonpharmacological treatment options, and one these commenters noted that collecting data on pain management strategies would ensure the highest effectiveness, lowest cost, and least invasive and addictive modalities are used in the treatment of chronic or subacute pain. One of these commenters supported the concept but also encouraged CMS to use the Centers for Disease Control and Prevention (CDC) Clinical Practice Guideline for Prescribing Opioids for Pain [88] as some SNF residents may appropriately need these medications, suggesting that there are key populations that should be excluded from any measures that could reduce their access to these medications. Another one of these commenters stated that they were hopeful that the recently implemented MDS items in section J0300-J0600 which assesses pain interference with daily activities, sleep, and participation in therapy could provide a foundation for future proposed measures, if it can overcome the potential to incentivize inappropriate use of pain medication. They also noted that one of the largest challenges in the nursing facility environment is the high proportion of residents with cognitive deficits who may be unable to effectively verbalize pain responses. This commenter urged CMS to consider the fact that these residents may convey pain in other ways including gestures, vocalizations, or atypical behaviors and to consider how these residents could be incorporated into a future pain measure.
One commenter opposed the measure concept, stating that pain management is a challenging topic to address, including in the SNF, and a SNF's goal is to manage the resident's pain and discomfort. This commenter and others opposed the idea of a SNF QRP measure that included an expectation of an improvement in pain since it could unintentionally incentivize providers to lower resident pain levels by prescribing medications, including opioids. One of these commenters suggested that improving care and treatment for mental health substance use disorders would be a better use of resources in the SNF QRP.
3. Depression
Comment: We received several comments on the concept of depression for a future SNF QRP measure, and many commenters supported the concept. One of these commenters noted that identifying a resident's risk of depression early and implementing interventions to address depression in the SNF setting can help to improve overall resident outcomes and quality of life. Another one of these commenters encouraged CMS to pursue development of this measure as part of larger equity efforts within the program. Another one of these commenters agreed, noting that mental health parity and access policies are grounded in the health equity view that mental and behavioral health treatment, access, and coverage should be the same as for physical healthcare.
One commenter, who supported the measure concept, also noted that groundwork is needed to identify the importance, relevance, appropriateness, feasibility, and applicability of such a measure or measures. This commenter noted that the MDS has two resident mood screening tools, the Patient Health Questionnaire (PHQ)-2 to 9 (PHQ-2 to 9) and the Staff Assessment of Resident Mood PHQ-9-OV,[89] creating challenges with the data that would need to be considered if a depression quality measure were developed using both MDS-based resident mood depression screening tools. Another one of these commenters recommended that CMS develop a measure that reports the number of residents who are identified as having depression and then receive follow up care, stating that recognizing when SNF's provide care to such residents would be more meaningful than a measure that simply reports the number of residents with depression.
Two commenters opposed the measure concept of depression, noting that a measure may require SNFs to have additional resources to treat depression, to which they may not have access. One of these commenters noted that they already collect information and use physician documentation to identify mental health or other behavioral health issues, stating that adding another screening requirement would not improve the quality of care, but it would add cost and burden to the SNF clinical team.
4. Patient Experience of Care/Patient Satisfaction
Comment: We received many comments on the concept of a patient experience of care/patient satisfaction measure, and all commenters supported the idea of further development. One commenter noted that the lack of a patient experience of care/patient satisfaction measure is a notable gap in quality measurement and patient reported measures should be given equal consideration as data driven measures in the SNF QRP. Two commenters called patient self-report the gold standard to assess care quality, while another one recommended that patient experience measures include a focus on activities that have a meaningful impact on function rather than emphasizing activities that may be appealing to residents and caregivers, but do not support improvement of function.
Two commenters noted the value in a patient experience of care/patient satisfaction measure; specifically, noting that persons who believe their personal goals, care preferences, and priorities (GPP) are heard and followed-up on by the care team applying a person-centered approach are more likely to participate in their environment, be happier, and have better clinical outcomes. One of these commenters also encouraged CMS to look at the activities of the Moving Forward coalition in this area.
Two commenters made recommendations for a patient satisfaction measure, like the CoreQ, or a patient experience measure, such as the Consumer Assessment of Healthcare Providers and Systems (CAHPS), while several other commenters made recommendations for the type of questions that should be included, the number of questions a survey should have, how it should be completed, potential submission methods, exclusion criteria, psychometric properties, and CBE endorsement status.
5. Other Suggestions for Future Measure Concepts
Comment: In addition to comments received on the four measure concepts of pain, depression, vaccination, and patient experience of care/patient satisfaction, we also received a couple of comments urging careful consideration of the feedback CMS receives to ensure that future proposals account for the additional burden on providers, evaluate the operational impact on SNFs, and minimize the risk of gaming or inappropriately influencing performance results. Some commenters also made suggestions for future measure concepts for the SNF QRP.
One commenter suggested we consider measures that assessed management of degenerative cognitive conditions, effectiveness of disposition planning and care transitions, changes in resident function, rates of follow-up care, and residents' access to appropriate treatments and medications. Another commenter recommended measures related to timely and appropriate referral to hospice, advance care planning, and palliative care access and utilization. One commenter recommended developing a measure addressing needs navigation, utilizing the new Principal Illness Navigation (PIN) codes adopted in the 2025 Physician Fee Schedule,[90] to provide insight into the type of residents receiving these services and its utilization, while another commenter recommended the Patient Active Measure (PAM®) instrument [91] be added to the MDS or required in parallel to the MDS.
Response: We thank all the commenters for responding to this RFI. While we are not responding to specific comments in response to the RFI in this final rule, we will take this feedback into consideration for our future measure development efforts for the SNF QRP.
E. Form, Manner, and Timing of Data Submission Under the SNF QRP
1. Background
We refer readers to the current regulatory text at § 413.360(b) for information regarding the policies for reporting specified data for the SNF QRP.
2. Reporting Schedule for the New Standardized Patient Assessment Data Elements, and the Modified Transportation Data Element, Beginning October 1, 2025, for the FY 2027 SNF QRP
As outlined in sections VI.C.3. and VI.C.5. of the proposed rule, we proposed to adopt four new items as standardized patient assessment data elements under the SDOH category (one Living Situation item, two Food items, and one Utilities item) and to modify the Transportation standardized patient assessment data element previously adopted under the SDOH category beginning with the FY 2027 SNF QRP.
We proposed that SNFs would be required to report these new items and the modified Transportation item using the MDS beginning with residents admitted on October 1, 2025, through December 31, 2025, for purposes of the FY 2027 SNF QRP. Starting in CY 2026, we proposed that SNFs would be required to submit data for the entire calendar year for each program year.
We also proposed that SNFs that submit the Living Situation, Food, and Utilities items with respect to admission only would be deemed to have submitted those items with respect to both admission and discharge. We proposed that SNFs would be required to submit these four items at admission only (and not at discharge) because it is unlikely that the assessment of those items at admission would differ from the assessment of the same item at discharge. This will align the data collection for these proposed items with other SDOH items (that is, Race, Ethnicity, Preferred Language, and Interpreter Services) which are only collected at admission.[92] A draft of the proposed items is available in the Downloads section of the SNF QRP Measures and Technical Information web page at https://www.cms.gov/medicare/quality/snf-quality-reporting-program/measures-and-technical-information.
As we noted in section VI.C.5 of the proposed rule and in section VII.C.6 of this final rule, we continually assess the implementation of the new SDOH items, including A1250. Transportation, as part of our routine item and measure monitoring work. We received feedback from interested parties in response to the FY 2020 SNF PPS proposed rule (84 FR 17676 through 17678) noting their concern with the burden of collecting the Transportation item at admission and discharge. Specifically, commenters stated that a resident's access to transportation is unlikely to change between admission and discharge. We analyzed the data SNFs reported from October 1, 2023, through December 31, 2023 (Quarter 4 of CY 2023), and found that residents' responses do not significantly change from admission to discharge.[93] Specifically, the proportion of residents [94] who responded “Yes” to the Transportation item at admission versus at discharge differed by only 0.60 percentage points during this period. We find these results convincing, and therefore we proposed to require SNFs to collect and submit the modified standardized patient assessment data element, Transportation, at admission only.
We solicited public comment on our proposal to collect data on the following items proposed as standardized patient assessment data elements under the SDOH category at admission only beginning with October 1, 2025, SNF admissions: (1) Living Situation as described in section VI.C.3(a) of the proposed rule; (2) Food as described in section VI.C.3(b) of the proposed rule; and (3) Utilities as described in section VI.C.3(c) of the proposed rule. We also solicited comment on our proposal to collect the modified standardized patient assessment data element, Transportation, at admission only beginning with October 1, 2025, SNF admissions as described in section VI.C.5 of the proposed rule.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: Several commenters supported the proposed collection of the SDOH assessment items once, upon admission, noting that this would mitigate the administrative burden of data collection and reduce redundancy. One commenter acknowledged CMS's internal analysis of the Transportation assessment item that demonstrated a less than one percent change in the assessment item response between admission and discharge.
Response: We appreciate the commenters' input on the timing of collecting the proposed SDOH assessment items. We continually assess the implementation of the new SDOH assessment items as part of our routine item and measure monitoring work, and when we identify an opportunity to improve data collection, we want to implement it. In the FY 2025 SNF proposed rule (89 FR 23468 through 23469), we proposed to collect these new and modified assessment items at admission only because we believe it is unlikely that the assessment of these items at admission would differ from the assessment of the same items at discharge. We are mindful of provider burden and appreciate the support from several commenters who agreed that collection at admission only, rather than at both admission and discharge, would mitigate the administrative burden of data collection on these new and modified assessment items.
Comment: One commenter recommended CMS collect the proposed new SDOH assessment items at discharge only, rather than at admission, to facilitate discharge planning. One commenter expressed concerns about data for the SDOH items being collected on every assessment, noting that responses will not change during the resident's stay.
Response: We believe that collecting the SDOH assessment items at discharge only would be too late for the SNF to act on the information if it so chooses. As we explained in our proposal, obtaining this information early in the resident's stay will ensure the SNF has information that it could use to inform how it cares for the resident and during the discharge planning processes.
Regarding the commenter who expressed concerns about collecting the proposed new and modified assessment items on every assessment, we did not propose that SNFs would collect these items on every assessment of a resident. Rather, we proposed that SNFs would be required to report these new assessment items and the modified Transportation item using the MDS beginning with residents admitted on October 1, 2025, through December 31, 2025, for purposes of the FY 2027 SNF QRP, and for the entire calendar year for each program year thereafter. We note the SNF QRP's reporting requirements currently only apply to residents receiving skilled care in a SNF covered by Medicare Part A.
Comment: Two commenters suggested that CMS offer the flexibility for SNFs to use SDOH data collected during the transition of care to the SNF or during the look-back period, rather than requiring its collection at admission. These commenters stated that they believed CMS' focus should be on how SDOH information is used in care planning and discharge planning, rather than requiring this information be obtained via a resident's verbal responses during the look-back period of the initial assessment.
Several commenters noted that CMS already collects many of the proposed SDOH assessment items from other health care providers, such as hospitals or other post-acute providers, prior to a SNF stay, and encouraged CMS to consider supporting data portability and screening interoperability across healthcare providers to avoid unnecessary duplication of screenings and assessments.
Response: We interpret these commenters to be suggesting that CMS should allow SNFs to obtain information collected in previous healthcare settings, rather than requiring SNFs to obtain this information from the resident upon the resident's admission to the SNF. Obtaining information about the Living Situation, Food, Utilities, and Transportation assessment items directly from the resident, sometimes called “hearing the resident's voice,” is more reliable and accurate than obtaining it from a health care provider that previously cared for the resident for several reasons: the SNF would not know whether it was collected from the resident or from a family member or other source; the SNF would not know how the SDOH domain was defined—for example, whether utilities included electricity, gas, oil, or water or only asked about electricity; and the SNF would not be able to determine whether the potential problem had been resolved since then. Most importantly, we believe that by asking the resident these questions at admission, it may prompt further discussion with the resident about their needs and help formulate an appropriate discharge care plan.
We also appreciate the statements from commenters encouraging CMS to support data portability and screening interoperability. As we noted in the FY 2023 SNF PPS final rule (87 FR 47503 and 47504), to further interoperability in post-acute care settings, CMS, and the Office of the National Coordinator for Health Information Technology (ONC) participate in the Post-Acute Care Interoperability Workgroup (PACIO) to facilitate collaboration with interested parties to develop Health Level Seven International® (HL7) Fast Healthcare Interoperability Resource® (FHIR) standards. These standards could support the exchange and reuse of patient assessment data derived from the post-acute care (PAC) setting assessment tools, such as the MDS, Inpatient Rehabilitation Facility—Patient Assessment Instrument (IRF-PAI), Long-Term Care Hospital (LTCH) Continuity Assessment Record and Evaluation (CARE) Data Set (LCDS), the Outcome and Assessment Information Set (OASIS) used by Home Health Agencies, and other sources. The CMS Data Element Library (DEL) continues to be updated and serves as a resource for PAC assessment data elements, as well as furthers CMS' goal of data standardization and interoperability. We acknowledge that there are still opportunities to advance these goals, and we will take these comments into consideration.
Comment: Several commenters offered suggestions or recommendations for guidance related to collecting the proposed SDOH assessment items. One commenter recommended that CMS include coding logic to allow skipping the Utilities assessment item if a resident indicated that they do not have a steady place to live, since it would be inappropriate to ask about utilities if a resident has no place to live.
Response: We appreciate all the comments we received about coding these proposed new and modified SDOH assessment items, including the Utilities assessment item. We proposed that SNFs would be required to collect and submit information on the four new assessment items, to have complete information. We do not agree that it would be inappropriate to ask about utilities just because a resident does not have a place to live at the time of the assessment. The resident may be living in temporary housing or a shelter, and gathering this information would still be important for their discharge planning.
Comment: Some commenters were also concerned that the proposed SDOH assessment items will be challenging for SNF residents to respond to, considering that many SNF residents have cognitive impairments or are more severely ill than the average Medicare beneficiary for whom the AHC HRSN Screening Tool was developed.
Response: We believe SNFs are accustomed to working with residents with very complex medical conditions, including multiple comorbidities, stroke, and cognitive decline, and we are confident in their ability to collect this data in a consistent manner. There are currently several resident interview assessment items on the MDS, and SNFs are accustomed to administering these questions to cognitively impaired patients.
We also plan to provide training resources in advance of the initial collection of the assessment items to ensure that SNFs have the tools necessary to administer the new SDOH assessment items and reduce the burden to SNFs in creating their own training resources. These training resources may include online learning modules, tip sheets, questions and answers documents, and/or recorded webinars and videos, and would be available to providers in mid-2025, allowing SNFs several months to ensure their staff take advantage of the learning opportunities.
Comment: Another commenter expressed concerns about collecting data on the Transportation assessment item from residents younger than 18 years old and recommended that CMS provide consideration for residents requiring special accommodations. Additionally, one commenter recommended that CMS consider a response option for SDOH assessment items that residents refuse to answer due to concerns about confidentiality or embarrassment.
Response: We are uncertain what the commenter's concerns are related to collecting the Transportation assessment item from residents younger than 18 years old, but we interpret the commenter to be concerned that these residents would be too young to provide a response or that these residents may be too young to have a driver's license, so the question would not be applicable to them.
In response to the first potential concern that residents would be too young to provide a response, we highlight that there is growing recognition of the need for effective screening methods for HRSNs in all patient populations, including pediatrics and adolescents. Children are especially vulnerable to HRSN, as poverty in childhood correlates to poor health outcomes.[95 96 97] Although there is no standardized protocol for screening in pediatric settings,[98] organizations like the American Academy of Pediatrics provide toolkits with suggestions for a screening protocol. Transportation has been identified by hospitals and clinics [99 100] that care for pediatric and adolescent patients as an important area to screen. One hospital system began using the AHC HRSN Screening Tool, including the proposed Transportation item, during selected well child visits at a Federally Qualified Health Center, and found the tool was feasible to administer and identified more than a third of patients with one or more HRSNs.[101]
In response to the second potential concern that the question would not be applicable to these residents because they may be too young to have a driver's license, we believe that even if a patient younger than 18 years old cannot drive themselves, they may rely on others, or they may use public transportation. As a result, they may still have transportation access needs that should be identified.
We interpret the second part of the comment to be recommending that we modify the response options to collect information about residents requiring special transportation accommodations. Although the proposal would require SNFs to collect the modified Transportation assessment item as described in section VII.E.2. of this final rule, such collection could potentially prompt the SNF to initiate conversations with its residents about their potential Transportation needs, such as special accommodations a resident may need to access transportation. Additionally, SNFs may seek to collect any additional information that they believe may be relevant to their resident population to inform their care and discharge planning process.
Comment: One commenter recommended that CMS consider a response option for SDOH assessment items that residents refuse to answer due to concerns about confidentiality or embarrassment.
Response: As described in sections VII.C.3.(a), VII.C.3.(b), VII.C.3.(c), and VII.C.5., each proposed new and modified SDOH item includes response options for those scenarios where a resident declines or is unable to provide information: (7) Resident declines to respond; and (8) Resident is unable to respond.
Comment: A few commenters recommended provide SNFs more flexibilities in collecting the new and modified SDOH assessment items. Two of these commenters suggested the use of interviews, paper, and electronic survey tools to administer the new and modified SDOH assessment items. One of these commenters also noted that many provider pre-admission processes now involve residents filling out pre-admission questionnaires via paper, mobile apps, or resident portals.
Response: We appreciate the commenters' input on the mechanism of collecting the new and modified SDOH assessment items. SNFs may use different methods to collect the information from the resident, as long as they are consistent with the coding guidance and defined look-back periods in the MDS RAI manual.
Comment: One commenter expressed confusion with how CMS planned to collect the proposed new SDOH assessment items, since the MDS does not currently ask these questions.
Response: As stated in section VI.E.2 of the proposed rule, we proposed adding these assessment items to a future version of the MDS and requiring SNFs to begin collecting the assessment items for residents admitted on or after October 1, 2025. A draft of the assessment items can be found on the SNF QRP Measures and Technical Information web page in the Downloads section at https://www.cms.gov/medicare/quality/snf-quality-reporting-program/measures-and-technical-information.
Comment: One commenter was concerned that SNFs would not be able to collect the data on admission without knowledge of whether a patient is expected to successfully rehabilitate and return home or would have to remain in the nursing home as a long-stay resident.
Response: We acknowledge that residents' needs may change through the course of their recovery in the SNF, but we also note that while the proposal would require the collection of the SDOH items at admission, we hope the questions would enable future conversations between the SNF and residents about their potential SDOH needs. As the commenter pointed out, it is important to think about the resident's living situation in the context at multiple points during their care journey, and collecting these items at admission would be an important first step to that process.
Comment: Some commenters were concerned that the proposed SDOH assessment items are not applicable to long-term residents receiving skilled care under their Medicare Part A fee-for-service benefit, but who have no plans to discharge back to the community. One commenter specifically stated that the Utilities and Food assessment items are not appropriate for these long-term residents because they reside in the nursing home prior to their SNF stay. Two commenters recommended that CMS consider adding a response option or a skip pattern for SNF residents who are expected to be a long-term nursing home resident, or for those who have resided in the facility during the 12-month look-back period.
Response: We interpret these comments to be discussing long-term residents of a nursing facility (NF) who become eligible for a SNF stay and who are also not expected to be discharged from the SNF to the community. If a resident has resided in a NF for at least 366 days prior to the initiation of a new SNF stay, we acknowledge that such long-term residents of the NF will have had the HRSNs that are the subject of the proposed SDOH assessment items addressed by the NF during the 12-month look-back period that applies to those items.
After consideration of these comments, we are finalizing a modification to the data specifications of the new and modified SDOH items so that they exclude any SNF residents who, immediately prior to their hospitalization that preceded a new SNF stay, resided in a NF for at least 366 continuous days. The SNF will not be required to ask the resident regarding their specific living situation, food, utilities, or transportation access during the 12-month look-back period because the NF was responsible for providing these needed services. We believe applying this criterion will decrease SNFs' burden of collecting these SDOH items from SNF residents who have received services from a NF for the entirety of the 12-month look-back period.
Comment: One commenter recommended we also require Medicare Advantage (MA) plans to collect and submit SDOH data. They contend that MA plans do not collect data on SDOH, but also make skilled coverage and discharge decisions for plan enrollees. As a result, SDOH data is not part of MA plans' decision-making process for discharge planning and SNFs often disagree with the discharge and coverage decisions issued by MA plans.
Response: We thank the commenter for their recommendation and acknowledge that MA plans have a role to play in advancing health equity. While this recommendation is outside the scope of this rulemaking, we will consider this feedback for future policymaking. we note the SNF QRP's reporting requirements currently only apply to residents receiving skilled care covered by Medicare Part A.
Comment: One commenter spoke about how they convened multiple interested parties to discuss the various social needs related screening measures and how quality measures and quality programs can best meet resident needs and policymakers' objectives. The result of the meeting was ten principles for adoption, updating, and implementing quality measures related to social needs, and they encouraged CMS to consider these principles in furthering SDOH-related policies within quality reporting and payment programs.
Response: We thank the commenter and note that we are not proposing measures related to screening for HRSNs. We will consider this feedback for future policymaking.
Comment: In response to the proposal to adopt two new Food assessment items, one commenter urged CMS to require or strongly encourage SNFs to immediately refer residents to social services to provide residents and caregivers information on post-discharge nutrition and food services (such as meal programs and oral nutrition supplement options); as well as create a post-discharge nutrition/food service plan to ensure services are provided as quickly as possible after discharge from the SNF.
Response: We did not propose to require SNFs to do anything specific with the information they obtain from the resident in response to the Food items. SNFs already are required to develop and implement an effective discharge planning process that focuses on the resident's discharge goals, the preparation of residents to be active partners and effectively transition them to post-discharge care, and the reduction of factors leading to preventable readmissions. We believe the proposed new SDOH assessment items have the potential to generate actionable data SNFs can use to implement effective discharge planning processes that can reduce the risk for negative outcomes such as hospital readmissions and admission to a nursing facility for long-term care. Given that SNFs must develop and implement an effective discharge planning process that ensures the discharge needs of each resident are identified, we believe collection of these new SDOH items will provide key information to SNFs to support effective discharge planning.
Comment: Another commenter described the ongoing burden of CMS' requirement for facilities to collect COVID-19 data. They noted the lack of appropriate technology to manage regulatory requirements necessitates the development of numerous internal processes, and implementing the necessary technology requires significant time and financial investment.
Response: This comment is out of scope for our proposals for the SNF QRP. We will take this feedback into consideration with future policy development work.
After careful consideration of the public comments we received, we are finalizing our proposal to require SNFs to collect and submit data on the following items adopted as standardized patient assessment data elements under the SDOH category at admission only beginning with October 1, 2025, SNF admissions: (1) Living Situation as described in section VII.C.3(a) of this final rule; (2) Food as described in section VII.C.3(b) of this final rule; and (3) Utilities as described in section VII.C.3(c) of this final rule. We are also finalizing our proposal to require SNFs to collect and submit the modified standardized patient assessment data element, Transportation, at admission only beginning with October 1, 2025, SNF admissions as described in section VII.C.5 of this final rule. However, we are finalizing a modification to the data specifications of the new and modified SDOH items so that they exclude any SNF residents who, immediately prior to their hospitalization that preceded a new SNF stay, resided in a NF for at least 366 continuous days. SNFs can monitor the MDS 3.0 Technical Information web page at https://www.cms.gov/medicare/quality/nursing-home-improvement/minimum-data-set-technical-information for updates.
3. Participation in a Validation Process Beginning With the FY 2027 SNF QRP
Section 1888(h)(12)(A) of the Act (as added by section 111(a)(4) of Division CC of the Consolidated Appropriations Act, 2021 (Pub. L. 116-260)) requires the Secretary to apply a process to validate data submitted under the SNF QRP. Accordingly, we proposed to require SNFs to participate in a validation process that would apply to data submitted using the MDS and SNF Medicare fee-for-service claims as a SNF QRP requirement beginning with the FY 2027 SNF QRP. We proposed to amend the regulation text at § 413.360.
We are also considering additional validation methods that may be appropriate to include in the future for the current measures submitted through the National Healthcare Safety Network (NHSN), as well as for other new measures we may consider for the program. Any updates to specific program requirements related to the validation process would be addressed through separate and future notice-and-comment rulemaking, as necessary.
(a) Participation in a Validation Process for Assessment-Based Measures
The MDS is a resident assessment instrument that SNFs must complete for all residents in a Medicare or Medicaid certified nursing facility, and for residents whose stay is covered under SNF PPS in a non-critical access hospital swing bed facility. The MDS includes the resident in the assessment process, and uses standard protocols used in other settings to improve clinical assessment and support the credibility of programs that rely on MDS, like the SNF QRP.[102]
We proposed to adopt a validation process for the SNF QRP that is similar to the validation process that we have adopted for the SNF Value-Based Purchasing (VBP) program in the FY 2024 SNF PPS final rule (88 FR 53323 through 53325) beginning with the FY 2027 SNF QRP. We proposed that this process would closely align with the validation process we have adopted for the SNF VBP program and would have the following elements:
- We proposed that our validation contractor would select, on an annual basis, up to 1,500 SNFs that submit at least one MDS record in the calendar year (CY) 3 years prior to the applicable FY SNF QRP. For example, for the FY 2027 SNF QRP, we would choose up to 1,500 SNFs that submitted at least one MDS record in CY 2024. We also proposed that the SNFs that are selected to participate in the SNF QRP validation for a program year would be the same SNFs that are randomly selected to participate in the SNF VBP validation process for the corresponding SNF VBP program year.
- We proposed that our validation contractor would request up to 10 medical records from each of the selected SNFs. Each SNF selected would only be required to submit records once in a fiscal year, for a maximum of 10 records for each SNF selected. To decrease the burden for the selected SNF, we proposed that the validation contractor would request that the SNFs submit the same medical records, at the same time, that are required from the same SNFs for purposes of the SNF VBP validation.
- We proposed that the selected SNFs would have the option to submit digital or paper copies of the requested medical records to the validation contractor and would be required to submit the medical records within 45 days of the date of the request (as documented on the request). If the validation contractor has not received the medical records within 30 days of the date of the request, the validation contractor would send the SNF a reminder in writing to inform the SNF that it must submit the requested medical records within 45 days of the date of the initial request.
We proposed that if a SNF does not submit the requested number of medical records within 45 days of the initial request, we would, under section 1888(e)(6)(A) of the Act, reduce the SNF's otherwise applicable annual market basket percentage update by 2 percentage points. The reduction would be applied to the payment update 2 fiscal years after the fiscal year for which the validation contractor requested records. For example, if the validation contractor requested records for FY 2027, and the SNF did not send them, we would reduce the SNF's otherwise applicable annual market basket percentage update by 2 percentage points for the FY 2029 SNF QRP.
We also stated that we intended to propose in future rulemaking the process by which we would evaluate the submitted medical records against the MDS to determine the accuracy of the MDS data that the SNF reported and that CMS used to calculate the measure results. We solicited public comment on what that process could include.
We solicited public comments on our proposal to require SNFs that participate in the SNF QRP to participate in a validation process for assessment-based measures beginning with the FY 2027 SNF QRP.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: Several commenters supported our proposal to require SNFs to participate in a validation process that would apply to data submitted using the MDS, and specifically to adopt a validation process for the SNF QRP that is similar to the validation process we have adopted for the SNF VBP program. Most of these commenters appreciated the fact that we proposed using the same process that was adopted for the SNF VBP program, and that records requested and submitted would apply to the validation processes for both the SNF QRP and SNF VBP, reducing provider burden.
Response: We agree that adopting a validation process for the SNF QRP that is similar to the validation process that we adopted for the SNF VBP program and using the same charts for both programs closely aligns the validation processes and reduces burden for SNFs.
Comment: Several commenters noted that SNFs are required to submit data for the SNF QRP and SNF VBP on different timelines and questioned how the same records could be used for both programs. Specifically, they pointed to the fact that SNFs submit data for the SNF QRP on a calendar year (CY) basis, whereas SNFs submit data for the SNF VBP on a fiscal year (FY) basis for purposes of both baseline and performance period calculations. These commenters requested that CMS resolve the apparent misalignment between the two programs' performance periods prior to finalizing the proposal.
Response: Our intent is to use the same records, to the extent feasible. However, we acknowledge that our proposal could have created confusion for SNFs.
Therefore, we are finalizing this proposal with modification to align the data collection period for the SNF QRP validation process with the SNF VBP validation process so that the requested charts will apply to the same FY program year for the SNF QRP and SNF VBP. Specifically, we are finalizing that our validation contractor will select, on an annual basis, up to 1,500 SNFs that submit at least one MDS record in the fiscal year (FY) 2 years prior to the applicable FY SNF QRP. For example, if the validation contractor requested records for FY 2025, and the SNF did not submit them 45 days of the initial request, we would reduce the SNF's otherwise applicable annual market basket percentage update by 2 percentage points for the FY 2027 SNF QRP (See Table 30). We are also finalizing conforming modifications to the regulation text at § 413.360(g)(1)(i), as discussed in section VII.E.3(c) of this final rule.
This change will not affect the data collection or data submission periods for the SNF QRP or the application of any reduction of the SNF's otherwise applicable APU for meeting the SNF QRP reporting requirements, including the required thresholds for the standardized patient assessment data collected using the MDS or the data collected and submitted through the CDC NHSN. This modification to our proposal to use a FY period from which to identify MDS for validation rather than a CY data collection period will only impact the new data validation process requirement. We acknowledge that this will result in SNFs having different data collection periods within the SNF QRP.
Therefore, if the validation contractor requested records for FY 2025, and the SNF did not submit them within 45 days of the initial request, the SNF would be found to be non-compliant with the SNF QRP requirements for the FY 2027 SNF QRP. SNFs will be notified through the already established methods if they are found to be non-compliant with the SNF QRP requirements, including this new validation process as finalized. Specifically, CMS issues notices of non-compliance to SNFs via a letter distributed through at least one of the following notification methods: the Non-Compliance Notification folders within the internet Quality Improvement and Evaluation System (iQIES), the United States Postal Service (USPS); or via an email from the SNFs Medicare Administrative Contractor. For more information on this process and timeline, see the SNF QRP Reconsideration and Exception & Extension web page at https://www.cms.gov/medicare/quality/snf-quality-reporting-program/reconsideration-and-exception-extension.
Comment: Commenters questioned how one chart could be used to validate data on measures that have different measure specifications in the SNF QRP versus the SNF VBP and provided an example. They noted that the SNF VBP program uses the Percent of Residents Experiencing One or More Falls with Major Injury (Long stay) measure which reports the percentage of long-stay nursing home residents with 101 or more cumulative days in the facility and had one or more falls with major injury reported, while the SNF QRP uses the Application of Percent of Residents Experiencing One or More Falls with Major Injury (Long stay) measure, which reports the percentage of Medicare Part A SNF stays during which one or more falls with major injury were reported.
Response: We understand that measures used in the SNF QRP and the SNF VBP program may have different measure specifications, including the measure noted by the commenters. For example, Resident C and Resident D were both residents of a SNF. Resident C was admitted to a SNF for 26 days and then was discharged to home. Resident D, however, had been a resident of a NF for 2 year and then received care as a hospital inpatient making them eligible for a SNF stay. After Resident D's hospital inpatient stay, they subsequently received skilled services at the same NF/SNF.
If the validation contractor requested the medical records for Resident C, the SNF would be subject to the 2 percentage penalty if they failed to submit the medical record for the validation process. If the validation contractor requested the medical records for Resident D, the SNF QRP measures related to Resident D skilled stay are subject to validation using the medical record and the SNF would be subject to the 2-percentage penalty if they failed to submit the medical record for the validation process. With respect to the SNF VBP program measures, Resident D's medical records would be used to validate the Percent of Residents Experiencing One or More Falls with Major Injury (Long stay) measure as required by the SNF VBP program validation process but will not be subject to the SNF QRP penalty for failure to submit the medical record. Any action for not submitting required medical records for the SNF VBP program that are not part of the SNF QRP program will be included in future rulemaking.
Comment: A commenter requested that CMS clarify that the 2 percentage point penalty would apply in total to both the SNF QRP and SNF VBP program data validation processes.
Response: The 2 percentage point penalty would apply to the SNF QRP only. There is currently no validation penalty in the SNF VBP.
Comment: A commenter requested that CMS clarify whether the 2 percentage point reduction to the applicable annual market basket update when a SNF does not submit the requested number of medical records within 45 days of the initial request is the same 2 percentage point reduction that would apply to a SNF who did not meet the reporting threshold, or whether there are two separate 2 percentage point penalties. they are concerned a SNF will be penalized for the same error in more than one way simultaneously, creating a double jeopardy.
Response: We interpret the commenter's reference to a reporting threshold to be referring to the data completion thresholds for reporting measures data and standardized patient assessment data collected using the MDS and the data collected and submitted through the NHSN. In section VI.E.3.(c) of the proposed rule, we proposed to add paragraph (f)(1)(iv) to our regulation at § 413.360 to establish that, if the SNF is selected for the validation process, the SNF must submit 100 percent of medical records requested (up to 10), in their entirety, within 45 days of the initial request. Failure to meet this proposed data completeness requirement (submitting medical records in their entirety as requested) or the required thresholds currently in place (for the standardized patient assessment data collected using the MDS or the data collected and submitted through the CDC NHSN) would result in application of the 2 percentage point penalty to the SNF only under the SNF QRP.
To summarize, we are finalizing that SNFs must comply with the validation process to avoid application of the 2 percent penalty under section 1888(e)(6)(A) of the Act. If the SNF fails to submit those medical records within 45 days of the date on the initial request, then we would apply the 2 percentage point penalty to FY 2027 SNF payments. We would not apply more than one penalty to a SNF for the same program year for failure to meet one or more of the SNF QRP's reporting requirements for that program year.
Comment: Two commenters suggested CMS extend the time period for SNFs to submit the medical records for data validation. One of these commenters suggested an extension to 60 days. The other commenter stated that only one written notification sent and one follow-up after 30 days was not adequate. They noted that written letters are easily misplaced, especially in facilities with administration turnover, and requested that CMS propose additional ways to notify providers of these reviews, including placing the request on the claim remittance.
Response: We disagree with the commenters and believe that 45 days with two notifications is the appropriate amount of notification. This is consistent with other auditing time periods for SNFs. For example, additional documentation requests (ADRs) sent by the Medicare Administrative Contractors, Special Medicare Review Contractors and Recovery Audit Contractors require records to be submitted within 45 days of the receipt of the letter.[103]
Comment: One commenter requested further clarification on the process by which a SNF would be notified they had been selected for a validation audit and how CMS would provide confirmation that the records had been received.
Response: SNFs selected for a validation audit will be notified via a letter sent through the internet Quality Improvement and Evaluation System (iQIES). We will notify SNFs that the medical records were received via a letter sent through iQIES or via email.
Comment: Several commenters stated they were concerned about the impact of a 2 percentage point payment adjustment to a randomly selected SNF that was required to submit documentation to support one MDS per year versus a randomly selected SNF that was required to submit documentation to support a maximum of 10 MDSs per year. These commenters stated that the risk of possibly dropping below an arbitrary threshold for a SNF that was required to submit documentation to support a maximum 10 MDS per year. They believe this barrier would be extremely difficult to overcome in a fair manner.
Response: In section VII.E.3.(a) of this final rule, we proposed that our validation contractor would request up to 10 medical records from each of the randomly selected SNFs. If a SNF is selected for the validation process and the SNF submits the requested number of medical records within 45 days of the date of the initial letter, then the SNF has met the proposed data completeness requirement for the validation process. While we acknowledge the highly unlikely scenario of a SNF being selected for validation on the basis of a single MDS submission during the relevant time period, we believe it is necessary to initially include all SNFs in the data validation process to meet the statutory requirement to implement a validation process for all data submitted for the SNF QRP.
We also noted in the same section of the rule that we intend to propose in future rulemaking the process by which we would evaluate the submitted medical records against the MDS to determine the accuracy of the MDS data that the SNF reported and that CMS would use to calculate the measure results (89 FR 23469). In establishing a validation threshold in future rulemaking, we will consider feedback about small sample sizes and/or uncertainty associated with sampling into account in our statistical approach.
Comment: Several commenters were concerned that our proposed timeline for implementation of the validation process for assessment-based measures in the FY 2027 SNF QRP year does not allow time for future rulemaking to determine the process by which we would evaluate the submitted medical records against the MDS, determine the accuracy of the MDS data the SNF reported, and provide subsequent notification to the provider in a timely manner that would allow for reconsideration requests, if needed.
They also stated they were concerned about a number of aspects of the validation process that CMS did not describe in the proposed rule, including the appeal process if a SNF disagreed with the validation contractor's findings, the expected threshold for compliance with the data validation, the penalty for noncompliance with the validation threshold, and the penalty for noncompliance with the validation threshold for the SNF VBP program. These commenters are concerned that if CMS establishes an arbitrary minimum MDS accuracy threshold for the SNF QRP validation process in the future without first establishing clear guidelines understood by both the providers and the SNF QRP validation contractors regarding support documentation requirements for each SNF QRP assessment-based element, there could be severe variation in the SNFs' performance scores. As a result, they believe that without clear guidelines the results of a validation audit would be dependent upon the SNF QRP validation contractor's independent determination rather than on whether the MDS was accurately completed per CMS requirements.
Response: Our proposal was limited to requiring SNFs that are selected for validation to submit the requested medical records and to impose a penalty if they do not comply with the request. Therefore, we believe that our proposed implementation timeline is reasonable. We intend to propose in future rulemaking a methodology for validating the submitted medical records against the MDS to determine the accuracy of the MDS data the SNF reported and CMS used to calculate the measure results.
Comment: One commenter recommended that CMS not sample the same facilities year over year if those facilities are performing well, but rather target low performers so as not to impose undue burden on facilities that are appropriately completing the MDS.
Response: We proposed to align the validation processes between the SNF QRP and SNF VBP programs to reduce the potential burden associated with the SNF QRP validation process. In the FY 2024 SNF PPS final rule (88 FR 53324 through 53325), CMS adopted a SNF VBP program validation process in which we would randomly select the SNFs to participate for the corresponding SNF VBP program year. However, we also recognize that SNFs would want an opportunity to provide input on potential criteria we would use in a targeted selection process as well as need ample notification regarding any targeted selection criteria. We will consider moving to a targeted selection process for future rulemaking.
We note that beginning with a random selection process and moving to a targeted selection process is consistent with the validation process for the Hospital IQR Program. We began with random selection of participating hospitals for the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) program (now the Hospital IQR Program) for the FY 2012 payment determination (74 FR 43884 through 43889). For the FY 2013 payment determination and subsequent years, we finalized the adoption of an initial targeting criterion after soliciting comments about potential targeting criteria (75 FR 50227 through 50229). As with the Hospital IQR Program's validation process, the SNF QRP will start with a random selection process and consider moving to a targeted selection process in future rulemaking. This is to ensure that we gain experience in auditing the MDS and the corresponding SNF medical records before we consider whether to propose a targeting methodology. We believe that this experience will ensure a fair and equitable audit process for all SNFs.
Comment: We received several comments related to the burden associated with the proposals for SNFs to participate in a validation process for assessment-based measures reported in the SNF QRP. Many of these commenters were appreciative of our efforts to reduce burden through using the same records for both SNF VBP validation and the SNF QRP validation. Three of these commenters noted it would reduce the risk of a SNF being audited in back-to-back validation cycles. Several commenters stated they opposed the 2 percentage point penalty reduction for failure to submit the requested medical records because SNFs cannot afford continued decreases in their payments, and the proposal would create additional administrative burden for SNFs that are already suffering staffing deficiencies. One of these commenters noted that adding validation audits is not effective in improving services in a SNF.
Response: We acknowledge the commenters' concerns regarding the potential burden associated with the proposals. We are aware of potential provider burden and carefully considered the options available to us to meet the statutory requirements while also mitigating provider burden. As we previously noted in section VI.E.3. of the proposed rule and section VII.E.3. of this final rule, section 1888(h)(12) of the Act requires that the Secretary apply a process to validate data submitted under the SNF QRP. In addition, we are interested in ensuring the validity of the data reported by SNFs because use of these data has public reporting implications under the SNF QRP. Valid and reliable quality measures are fundamental to the effectiveness of our quality reporting programs. To ensure we receive the medical records we request from selected SNFs, we proposed to require timely submission of requested medical records for the SNF QRP validation process. Specifically, we proposed to apply the SNF QRP's 2 percentage point reduction in accordance with section 1888(e)(6)(A) of the Act if the selected SNF failed to submit 100 percent of the requested medical records as specified. We believe these proposals will ensure we receive the requested medical records so we may validate the data they submitted for the SNF QRP.
Our goal is to minimize the burden we impose on SNFs under the SNF QRP and we will continue considering this topic as we explore proposing additional policies for the SNF QRP validation process. As discussed further in section VI.E.3.(b) of this rule, we note that the claims-based measures validation process we proposed does not impose any new burden on SNFs.
We invited public comments on the future process by which we would evaluate the submitted medical records against the MDS to determine the accuracy of the MDS data that the SNF reported and that CMS would use to calculate the measure results. We received several comments providing various recommendations in response to this request.
Comment: One commenter urged CMS to ensure the reviews are done in a fair and equitable manner, including having therapy professionals on the review team when therapy services are provided to validate the functional components associated with SNF QRP measures. Two commenters noted that when the MDS was initially developed it was intended to be a source record, particularly related to interview questions, and there was no need to document elsewhere in the medical record redundant assessment information. These commenters noted that as the MDS has become a tool for reimbursement purposes, payment auditors have penalized providers for not having this redundant documentation repeated in the medical record, and also note that some States have their own documentation requirements, sometimes contrasting with those requirements published in the MDS Resident Assessment Instrument (RAI) manual. Therefore, these commenters urged CMS to meet with SNFs, including hosting a technical expert panel. Several commenters urged CMS to have an appeals process SNFs could access if they disagree with the validation contractor's findings, and a process through which SNFs could apply for hardship exemption.
Finally, one commenter urged CMS to share this information as soon as possible and provide ample time for evaluation and feedback prior to finalizing and implementing a validation process to validate MDS accuracy.
Response: We thank the commenters for their suggestions, and we will consider this feedback as we consider future rulemaking.
After careful consideration of the public comments we received, we are finalizing this proposal with modification that SNFs that participate in the SNF QRP will be required to participate in a validation process for assessment-based measures beginning with the FY 2027 SNF QRP. Specifically, our validation contractor will select, on an annual basis, up to 1,500 SNFs that submit at least one MDS record in the FY two years prior (rather than the CY 3 years prior) to the applicable FY SNF QRP. For example, for the FY 2027 SNF QRP, we will choose up to 1,500 SNFs that submitted at least one MDS record in FY 2025.
(b) Application of the Existing Validation Process for Claims-Based Measures Reported in the SNF QRP
Beginning with the FY 2027 SNF QRP, we proposed to apply the process we currently use to ensure the accuracy of the Medicare fee-for-service claims to validate claims-based measures under the SNF QRP. Specifically, information reported through Medicare Part A fee-for-service claims are validated for accuracy by Medicare Administrative Contractors (MACs) to ensure accurate Medicare payments. MACs use software to determine whether billed services are medically necessary and should be covered by Medicare, review claims to identify any ambiguities or irregularities, and use a quality assurance process to help ensure quality and consistency in claim review and processing. They conduct prepayment and post-payment audits of Medicare claims, using both random selection and targeted reviews based on analyses of claims data.
We use data to calculate claims-based measures for the SNF QRP. We believe that adopting the MAC's existing process of validating claims for medical necessity through targeted and random audits would satisfy the statutory requirement to adopt a validation process for data submitted under the SNF QRP for claims-based measures at section 1888(h)(12)(A) of the Act (as added by section 111(a)(4) of Division CC of the Consolidated Appropriations Act, 2021 (Pub. L. 116-260)).
We solicited public comment on our proposal to apply the MAC's existing validation process for the SNF QRP claims-based measures beginning with the FY 2027 program year.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: Two commenters stated that the proposal was vague and provides insufficient detail to estimate what the scope and burden would be associated with this proposal. One commenter submitted a number of questions seeking clarification on the process for claims-based measure validation, including the number of SNF providers that would be subject to the proposed claims-based SNF QRP validation process, whether there was a limit to the number of claims for which a provider must submit supporting documentation to the MAC, what specific documentation would SNFs be required to submit to the MAC, the specific criteria fee-for-service payment contractors would use to validate the accuracy of the SNF quality-related data, and how a fee-for-service payment auditor would convert/apply their payment process to review claims. Finally, these commenters recommended CMS rescind this proposal and meet with interested parties to identify a more appropriate approach to be presented in subsequent rulemaking.
Response: We interpret the commenters to be seeking further clarification on several issues related to how claims would be validated. As we noted in section VI.E.3.(b) of the proposed rule and section VII.E.3.(b) of this final rule, we proposed to use the same process for the SNF QRP claims-based measures as we adopted in the FY 2023 SNF PPS final rule (87 FR 47590 through 47591) for the SNF All-Cause Readmission (SNFRM) measure in the SNF VBP, since many of SNF QRP measures have already been adopted into the SNF VBP program.
Specifically, we believe that relying on the MACs' existing process of validating claims for medical necessity through targeted and random audits, as discussed in our proposal, satisfies our statutory requirement to adopt a validation process for claims-based measures for the SNF QRP. Given that we calculate SNFs' performance on claims-based measures based on claims they submit for payment under Medicare Part A, and SNFs do not submit any additional data for these claims-based measures, the only information to be validated is whether the claim accurately reflects the services the SNF provided. The MACs' existing process for validating claims, including whether they are medically necessary, addresses whether the information in the claims, which we use to calculate the claim-based measures, is accurate. We also believe that using the same validation process will reduce any additional burden and mitigate any concerns from providers. On this basis, we proposed to rely on the MACs' existing claims validation process to validate the information we use to calculate claims-based measures for SNFs. We clarify that we would deem the information reported through claims, and used for claims-based measures, as validated based on the MACs' existing process for validating the accuracy of claims; neither SNFs nor CMS would take any further action to validate claims-based measures under this proposal. If we decide to further validate claims-based measures beyond the MAC's existing process, this would be done in future rulemaking.
Comment: Two other commenters questioned how CMS' process to validate claims for medical necessity is analogous to validating data for accuracy in quality reporting and requests further clarification.
Response: Specifically, we believe that relying on the MACs' existing process of validating claims for medical necessity through targeted and random audits, as discussed in our proposal, satisfies our statutory requirement to adopt a validation process for claims-based measures for the SNF QRP. Given that we calculate SNFs' performance on claims-based measures based on claims they submit for payment under Medicare Part A, and SNFs do not submit any additional data for these claims-based measures, the only information to be validated is whether the claim accurately reflects the services the SNF provided. The MACs' existing process for validating claims, including whether they are medically necessary, addresses whether the information in the claims, which we use to calculate the claim-based measures, is accurate. We also believe that using the same validation process will reduce any additional burden and mitigate any concerns from providers.
After careful consideration of the public comments we received, we are finalizing our proposal to apply the MAC's existing validation process for the SNF QRP claims-based measures beginning with the FY 2027 program year.
(c) Amending the Regulation Text at § 413.360
We proposed to amend our regulation at § 413.360 to reflect these proposed policies. Specifically, we proposed to add paragraph (g) to our regulation at § 413.360, which would codify the procedural requirements we proposed for these validation processes for SNF QRP. We also proposed to add paragraph (f)(1)(iv) to our regulation at § 413.360 to establish that, if the SNF is selected for the validation process, the SNF must submit up to 10 medical records requested, in their entirety. Finally, we proposed minor technical amendments for our regulation at § 413.360(f)(3) to apply to all data completion thresholds implemented in § 413.360(f)(1).
We solicited public comments on our proposal to amend our regulation at § 413.360. We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: We received one comment on our proposal to amend the regulation text at § 413.360. This commenter noted that in the proposed rule on display at the Federal Register (89 FR 23494 column 2), it appears that the proposed § 413.360(g)(1)(iii) may be misworded. Specifically, paragraph (g)(1)(iii) is under § 413.360(g), the description of MDS-assessment-based SNF QRP validation process requirement to submit supporting medical records documentation within 45 days of the date of the records request. However, it refers to paragraph (g)(2) which is related to the claims-based SNF QRP validation process, rather than referencing the MDS-based validation process paragraph (g)(1).
Response: We thank the commenter for pointing out this typographical error. We are finalizing § 413.360(g)(1)(iii) with modification to correct this minor technical error.
Comment: We received one comment on our proposal to add the regulation text at § 413.360(g)(2). This commenter requested that paragraph (g)(2) should be rescinded from the proposed 413.360 revisions pending further consideration for reintroduction in a revised manner in future rulemaking.
Response: We disagree with the commenter. As we noted in section VI.E.3.(b) of the proposed rule and section VII.E.3.(b) of this final rule, we proposed to use the same process for the SNF QRP claims-based measures as we adopted in the FY 2023 SNF PPS final rule (87 FR 47590 through 47591) for the SNF All-Cause Readmission (SNFRM) measure in the SNF VBP, since many of SNF QRP measures have already been adopted into the SNF VBP program.
Specifically, we believe that relying on the MACs' existing process of validating claims for medical necessity through targeted and random audits, as discussed in our proposal, satisfies our statutory requirement to adopt a validation process for claims-based measures for the SNF QRP. Given that we calculate SNFs' performance on claims-based measures based on claims they submit for payment under Medicare Part A, and SNFs do not submit any additional data for these claims-based measures, the only information to be validated is whether the claim accurately reflects the services the SNF provided. The MACs' existing process for validating claims, including whether they are medically necessary, addresses whether the information in the claims, which we use to calculate the claim-based measures, is accurate. We also believe that using the same validation process will reduce any additional burden and mitigate any concerns from providers. On this basis, we proposed to rely on the MACs' existing claims validation process to validate the information we use to calculate claims-based measures for SNFs. We clarify that we would deem the information reported through claims, and used for claims-based measures, as validated based on the MACs' existing process for validating the accuracy of claims; neither SNFs nor CMS would take any further action to validate claims-based measures under this proposal. If we decide to further validate claims-based measures beyond the MAC's existing process, this would be done in future rulemaking.
Comment: We received one comment related to SNF QRP data collected and submitted through NHSN that was out of scope of the proposals for the SNF QRP assessment-based measures and claims-based measures validation processes. This commenter requested CMS to engage with SNF interested parties in potential future additional SNF QRP validation approaches related to data submitted through NHSN. They note there have been multiple challenges for providers over the years with both the data submission processes to NHSN as well as data coordination between the CDC that manages NHSN reporting processes, and CMS who manages the SNF QRP requirements.
Response: This comment is out of scope for our proposals for the SNF QRP. We will take the commenter's request into consideration for our future policy making with respect to the validation process.
After careful consideration of the public comments we received, we are finalizing our proposal to amend our regulation at § 413.360 to codify the data validation process for the SNF QRP with two modifications. First, as discussed in section VII.E.3.(a) of this final rule, we are finalizing our proposal for selection of SNFs for this validation process with modification. We are finalizing that our validation contractor will select, on an annual basis, up to 1,500 SNFs that submit at least one MDS record in the FY 2 years prior, rather than the CY 3 years prior, to the applicable FY SNF QRP. Therefore, we are finalizing the regulation text at § 413.360(g)(1)(i) with modification to conform with this modification to our criteria for selecting SNFs to participate in this validation process.
Second, we are modifying the regulation text at § 413.360(g)(1)(iii) to correct a minor technical error, so it properly cross-references paragraph (g)(1) instead of paragraph (g)(2).
F. Policies Regarding Public Display of Measure Data for the SNF QRP
As outlined in the proposed rule, we did not propose any new policies regarding the public display of measure data in the FY 2025 SNF PPS proposed rule. For a discussion of our policies regarding public display of SNF QRP measure data and procedures for the SNFs to review and correct data and information prior to their publication, we refer readers to the FY 2017 SNF PPS final rule (81 FR 52045 through 52048).
VIII. Updates to the Skilled Nursing Facility Value-Based Purchasing (SNF VBP) Program
A. Statutory Background
Through the Skilled Nursing Facility Value-Based Purchasing (SNF VBP) Program, we award incentive payments to SNFs to encourage improvements in the quality of care provided to Medicare beneficiaries. The SNF VBP Program is authorized by section 1888(h) of the Act, and it applies to freestanding SNFs, SNFs affiliated with acute care facilities, and all non-CAH swing bed rural hospitals. We believe the SNF VBP Program has helped to transform how Medicare payment is made for SNF care, moving increasingly towards rewarding better value and outcomes instead of merely rewarding volume. Our codified policies for the SNF VBP Program can be found in our regulations at 42 CFR 413.337(f) and 413.338.
1. Spotlight on the CMS National Quality Strategy
As part of the CMS National Quality Strategy,[104] we are committed to aligning measures across our quality programs and ensuring we measure quality across the entire care continuum in a way that promotes the best, safest, and most equitable care for all individuals.
We believe that improving alignment of measures across the CMS quality programs will reduce provider burden while also improving the effectiveness of quality programs. However, we also recognize that a one-size-fits-all approach fails to capture important aspects of quality in our healthcare system across populations and care settings.
To move towards a more streamlined approach that does not lose sight of important aspects of quality, we are implementing a building-block approach: a “Universal Foundation” of quality measures across as many of our quality reporting and value-based care programs as possible, with additional measures added on depending on the population or setting (“add-on sets”).[105]
Our goal with the Universal Foundation is to focus provider attention on measures that are the most meaningful for patients and patient outcomes, reduce provider burden by streamlining and aligning measures, allow for consistent stratification of measures to identify disparities in care between and among populations, accelerate the transition to interoperable, digital quality measures, and allow for comparisons across quality and value-based care programs to better understand what drives quality improvement and what does not.
We select measures for the Universal Foundation that are of high national impact, can be benchmarked nationally and globally, are applicable to multiple populations and settings, are appropriate for stratification to identify disparity gaps, have scientific acceptability, support the transition to digital measurement, and have no anticipated unintended consequences with widespread measure implementation.
We believe that the creation of this Universal Foundation will result in higher quality care for the more than 150 million Americans covered by our programs and will serve as an alignment standard for the rest of the healthcare system. We continue to collect feedback from interested parties through listening sessions, requests for information and proposed rulemaking, and other interactions to refine our approach as we work to implement the Universal Foundation across our quality programs. As we continue building the SNF VBP measure set, we intend to align with the measures in the Universal Foundation, as well as the post-acute care add-on measure set, to the extent feasible.
We received one comment on our discussion of the CMS National Quality Strategy. The following is a summary of the comment we received and our response.
Comment: One commenter supported CMS' intent to align the Program's measure set with the Universal Foundation.
Response: We thank the commenter for their support.
B. Regulation Text Technical Updates
We proposed to make several technical updates to our regulation text. First, we proposed to update § 413.337(f) to correct the cross-references in that section to § 413.338(a). Second, we proposed to update the definition of “SNF readmission measure” in § 413.338(a) by replacing the references to the Skilled Nursing Facility Potentially Preventable Readmissions (SNFPPR) measure with a reference to the Skilled Nursing Facility Within-Stay Potentially Preventable Readmission (SNF WS PPR) measure, by clarifying that we specified both measures under section 1888(g) of the Act, and by clarifying that the SNF readmission measure will be the SNF WS PPR measure beginning October 1, 2027. This change will align the definition of “SNF readmission measure” with policies we have previously finalized for the SNF VBP, including that we will not use the SNFPPR and that we will replace the SNFRM with the SNF WS PPR beginning October 1, 2027.
In addition, we proposed to redesignate the term “performance score” at § 413.338(a) with the term “SNF performance score” for consistency with the terminology we are now using in the Program, and to make conforming edits to the last sentence of § 413.337(f). We also proposed to replace the references to “program year” with “fiscal year” in the definitions of “health equity adjustment (HEA) bonus points,” “measure performance scaler”, “top tier performing SNF”, and “underserved multiplier” to align the terminology with that used in the remainder of that section.
We also proposed to update § 413.338(f) to redesignate paragraphs (f)(1) through (4) as paragraphs (f)(2) through (5), respectively. We also proposed to add a new paragraph (f)(1) and to revise the newly redesignated paragraphs (f)(2) and (3).
In addition, we proposed to update § 413.338(j)(3) to include additional components of the MDS validation process that we finalized in the FY 2024 SNF PPS final rule (88 FR 53324 through 53325). In particular, we proposed to include the SNF selection, medical record request, and medical record submission processes for MDS validation.
Further, we proposed to remove § 413.338(d)(5) from the regulation text because the only measure that will be in the SNF VBP Program until the FY 2026 program year is the SNFRM, and to add new paragraph (l)(1) which will state that the SNF VBP measure set for each year includes the statutorily-required SNF readmission measure and, beginning with the FY 2026 program year, up to nine additional measures specified by CMS.
We invited public comment on these proposed technical updates to our regulation text.
We did not receive public comments on these proposals, and therefore, we are finalizing them as proposed.
C. SNF VBP Program Measures
1. Background
We refer readers to the FY 2024 SNF PPS final rule for background on the measures we have adopted for the SNF VBP Program (88 FR 53276 through 53297).
Table 31 lists the measures that have been adopted for the SNF VBP Program, along with their timeline for inclusion.
2. Measure Selection, Retention, and Removal Policy Beginning With the FY 2026 SNF VBP Program Year
Section 1888(h)(2) of the Act requires the Secretary to apply the measure specified under section 1888(g)(1) (currently the SNFRM) and replace that measure, as soon as practicable, with the measure specified under section 1888(g)(2) (currently the SNF WS PPR measure). Section 1888(h)(2) of the Act also allows the Secretary to apply, as appropriate, up to nine additional measures to the SNF VBP Program, in addition to the statutorily required SNF readmission measure. We have now adopted seven additional measures for the Program (see the FY 2023 SNF PPS final rule (87 FR 47564 through 47580) and the FY 2024 SNF PPS final rule (88 FR 53280 through 53296)).
Now that the SNF VBP Program includes measures in addition to the SNFRM (which will be replaced with the SNF WS PPR measure beginning with the FY 2028 program year), we stated in the FY 2025 SNF PPS proposed rule (89 FR 23471 through 23472) that we believe it is appropriate to adopt a policy that governs the retention of measures in the Program, as well as criteria we will use to consider whether a measure should be removed from the Program. These policies will help ensure that the Program's measure set remains focused on the best and most appropriate metrics for assessing care quality in the SNF setting. We also believe that the proposed measure removal policy will streamline the rulemaking process by providing a sub-regulatory process that we can utilize to remove measures from the Program that raise safety concerns while also providing sufficient opportunities for the public to consider, and provide input on, future proposals to remove a measure.
Other CMS quality programs, including the SNF QRP and Hospital Inpatient Quality Reporting (IQR) Program, have adopted similar policies. For example, in the FY 2016 SNF PPS final rule (80 FR 46431 through 46432), the SNF QRP adopted 7 removal factors and, in the FY 2019 SNF PPS final rule (83 FR 39267 through 39269), the SNF QRP adopted an additional measure removal factor, such that a total of eight measure removal factors are now used to determine whether a measure should be removed. The SNF QRP also codified those factors at § 413.360(b)(2).
For the purposes of the SNF VBP Program, we proposed to adopt a measure selection, retention, and removal policy beginning with the FY 2026 SNF VBP program year. The proposed policy would apply to all SNF VBP measures except for the SNF readmission measure because we are statutorily required to retain that measure in the measure set.
First, we proposed that when we adopt a measure for the SNF VBP Program for a particular program year, that measure will be automatically retained for all subsequent program years unless we propose to remove or replace the measure. We believe that this policy will make clear that when we adopt a measure for the SNF VBP Program, we intend to include that measure in all subsequent program years. This policy will also avoid the need to continuously propose a measure for subsequent program years.
Second, we proposed that we will use notice and comment rulemaking to remove or replace a measure in the SNF VBP Program to allow for public comment. We also proposed that we will use the following measure removal factors to determine whether a measure should be considered for removal or replacement:
(1) SNF performance on the measure is so high and unvarying that meaningful distinctions and improvements in performance can no longer be made;
(2) Performance and improvement on a measure do not result in better resident outcomes;
(3) A measure no longer aligns with current clinical guidelines or practices;
(4) A more broadly applicable measure for the particular topic is available;
(5) A measure that is more proximal in time to the desired resident outcomes for the particular topic is available;
(6) A measure that is more strongly associated with the desired resident outcomes for the particular topic is available;
(7) The collection or public reporting of a measure leads to negative unintended consequences other than resident harm; and
(8) The costs associated with a measure outweigh the benefit of its continued use in the Program.
Each of these measure removal factors represent instances where the continued use of a measure in the Program would not support the Program's objective, which is to incentivize improvements in quality of care by linking SNF payments to performance on quality measures. Therefore, we believe that these are appropriate criteria for determining whether a measure should be removed or replaced.
Third, upon a determination by CMS that the continued requirement for SNFs to submit data on a measure raises specific resident safety concerns, we proposed that we may elect to immediately remove the measure from the SNF VBP measure set. Upon removal of the measure, we will provide notice to SNFs and the public, along with a statement of the specific patient safety concerns that will be raised if SNFs continue to submit data on the measure. We will also provide notice of the removal in the Federal Register .
We proposed to codify this policy at § 413.338(l)(2) and (3) of our regulations.
We invited public comment on the proposed measure selection, retention, and removal policy. We also invited public comment on our proposal to codify this policy at § 413.338(l)(2) and (3).
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: Many commenters supported CMS' proposal to adopt a measure selection, retention, and removal policy. A few commenters appreciated that the policy aligns with the policies used in other CMS quality programs. A few commenters believed this policy allows CMS to prioritize evidence-based quality measures that are focused on critical aspects of quality and helps reduce the provider burden associated with data collection when a measure that is no longer valuable is removed from the Program. A few commenters supported the proposal to use notice and comment rulemaking to propose removal or replacement of a measure as well as to provide public notification when a measure is removed. One commenter supported the measure removal criteria believing that these criteria should be met before a measure is removed from the Program. One commenter believed this policy provides CMS flexibility to remove measures with safety concerns, which the commenter believed is important for maintaining high standards of care. One commenter believed this policy aligns with the criteria used by the Consensus-Based Entity (CBE) during the measure endorsement process.
Response: We thank the commenters for their support. We agree that this policy will help ensure that the Program's measure set remains focused on the best and most appropriate metrics for assessing care quality in the SNF setting.
Comment: A few commenters supported the measure selection, retention, and removal policy but also provided recommendations related to the proposed policy. One commenter encouraged CMS to seek input from interested parties when deciding to remove a measure based on measure removal factor 8 (the costs associated with a measure outweigh the benefit of its continued use in the Program) because the cost/benefit relationship may be viewed differently by different interested parties. One commenter recommended that CMS create publicly available monitoring reports that assess whether a measure shows or lacks meaningful performance improvement because many factors influence the threshold for determining when facilities can no longer make improvements, and the commenter believed it is important for the industry to understand these changes over time. One commenter recommended that CMS consider the correlation between existing SNF VBP measures and alternative metrics as part of the measure selection, retention, and removal policy. The commenter believed that if the correlation for the same desired outcome between the measures is high, CMS should also consider the measure for removal.
Response: We thank the commenters for their recommendations. With respect to the commenter's recommendation that we seek input from interested parties when deciding to remove a measure based on measure removal factor 8, we proposed to use notice and comment rulemaking to remove or replace a measure in the SNF VBP Program unless we determine that the continued requirement for SNFs to submit data on a measure raises specific resident safety concerns. We believe this proposal provides ample opportunity for interested parties to provide input. With respect to commenters' other recommendations, we intend to take these into consideration as part of our normal monitoring and evaluation efforts related to SNF VBP Program policies.
Comment: One commenter recommended that measures not endorsed by the CBE be removed and considered ineligible for inclusion in the SNF VBP Program.
Response: Although section 1888(h) of the Act does not require that measures adopted in the SNF VBP Program be endorsed by the CBE, we consider CBE-endorsed measures when selecting new measures to propose for the Program. In some cases, there is not a CBE-endorsed measure for a measure topic that we consider important for inclusion in the SNF VBP Program. For example, the Nursing Staff Turnover measure that we adopted in the FY 2024 SNF PPS final rule (89 FR 53281 through 53286) is not endorsed by the CBE, but we believe this measure is important for the SNF VBP Program given the well-documented impact of nursing staff turnover on resident outcomes.
Comment: One commenter did not support CMS' proposal to immediately remove a measure that raises resident safety concerns because it was not clear to the commenter how CMS would assess and make such a determination. The commenter also believed that this policy would give CMS the ability to make immediate decisions on removing measures without public input and without explaining to the public how the determination was made.
Response: We acknowledge the commenter's concern. We note that this proposed SNF VBP policy to immediately remove a measure that raises resident safety concerns is based on the policies finalized in other Programs such as the SNF QRP, which finalized this policy in the FY 2016 SNF PPS final rule (80 FR 46431), and the Hospital Value-Based Purchasing Program, which finalized this policy in the FY 2017 IPPS/LTCH PPS final rule (83 FR 41446). We intend to use this proposed authority narrowly and only in those circumstances where continued reporting on a measure poses specific and serious resident safety concerns. When making such a determination, we intend to review and analyze the available evidence raising a specific and serious resident safety concern and be transparent about our concerns and findings when the measure is removed and during subsequent rulemaking. For example, we announced in December 2008 that we would immediately remove the AMI-6-Beta blockers at arrival measure from the Hospital IQR Program (then known as the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) Program) following the release of updated clinical guidance and evidence of increased mortality risk for some patients. We subsequently confirmed the removal of the AMI-6-Beta blockers at arrival measure in the FY 2010 IPPS final rule (74 FR 43863). We also note that since we first adopted a version of this policy in FY 2010, we have applied the policy only sparingly.
Further, as stated in the proposed rule (89 FR 23472), if we elect to immediately remove a measure from the Program, we will provide notice to SNFs and the public through regular communication channels, along with a statement of the specific resident safety concerns that result from the continued use of the measure in the Program. We will also provide notice of the removal in the Federal Register .
After consideration of public comments, we are finalizing the measure selection, retention, and removal policy beginning with the FY 2026 program year as proposed. We are also finalizing our proposal to codify this policy at § 413.338(l)(2) and (3) of our regulations.
3. Future Measure Considerations
Section 1888(h)(2) of the Act allows the Secretary to apply, as appropriate, up to nine additional measures to the SNF VBP Program, in addition to the statutorily required SNF readmission measure. These measures may include measures of functional status, patient safety, care coordination, or patient experience.
In the FY 2022 SNF PPS proposed rule (86 FR 20009 through 20011), we requested public comment on potential future measures to include in the expanded SNF VBP Program. After considering the public input we received, we adopted three new measures in the FY 2023 SNF PPS final rule (87 FR 47564 through 47580). Two of those measures will be scored beginning with the FY 2026 program year: the SNF HAI and Total Nurse Staffing measures; and the third measure will be scored beginning with the FY 2027 program year: the DTC PAC SNF measure. In the FY 2024 SNF PPS final rule (88 FR 53280 through 53296), we adopted four additional measures. One of those measures, the Nursing Staff Turnover measure, will be scored beginning with the FY 2026 program year, while the other three measures will be scored beginning with the FY 2027 program year: the Falls with Major Injury (Long Stay), DC Function, and Long Stay Hospitalizations measures.
With the adoption of those seven measures, in addition to the statutorily required SNF readmission measure, the SNF VBP Program will include eight measures that cover a range of quality measure topics important for assessing the quality of care in the SNF setting. Therefore, as permitted under section 1888(h)(2)(A)(ii) of the Act, we can add up to two additional measures in the Program unless and until we remove measures in the future.
As part of our efforts to build a robust measure set for the SNF VBP Program, we are considering several options related to new measures and other measure set adjustments. First, we recognize that gaps remain in the current measure set and therefore, we are considering which measures are best suited to fill those gaps. Specifically, we are assessing several resident experience measures to determine their appropriateness and feasibility for inclusion in the Program. We are also testing the appropriateness of measures that address other CMS priorities, such as interoperability and health equity/social determinants of health.
Beyond the adoption of new measures, we are also considering other measure set adjustments. For example, we are assessing the feasibility of a staffing composite measure that would combine the two previously adopted staffing measures. We are also considering whether measure domains and domain weighting are appropriate for the SNF VBP Program.
While we did not propose any new measures or measure set adjustments in the proposed rule, we will continue to assess and determine which, if any, of these options would help us maximize the impact of the SNF VBP Program measure set and further incentivize quality of care improvements in the SNF setting. We welcomed commenters' continuing feedback on potential new measure topics and other measure set adjustments.
We received public comments related to future measure considerations for the SNF VBP Program. The following is a summary of the comments we received.
Comment: Several commenters supported CMS' consideration of an interoperability measure for the SNF VBP Program. Specifically, a few commenters recommended that a potential future interoperability measure assess electronic exchange of data elements critical to care transitions and that the measure be aligned with other Federal policies on this topic. A few commenters also recommended that any future measure on interoperability be paired with financial resources or other assistance to support the adoption of electronic health records (EHRs) and other health information technology (IT) resources in the SNF setting, and that CMS provide a transition period of 3 to 5 years for facilities to incorporate these technologies. One commenter suggested exploring interoperability measures to enable more consistent care across various health settings. One commenter recommended testing the interoperability measure prior to inclusion in the Program.
A few commenters expressed support for the potential future adoption of a resident experience measure noting that resident experience is a key measure of a provider's quality and that the lack of such a measure is the largest gap in the current SNF VBP measure set. One commenter recommended adoption of the CoreQ measure as it is a measure of resident satisfaction endorsed by the CBE. Another commenter recommended that CMS consider the Patient Activation Measure® performance measure (PAM-PM) for future application in the Program.
A few commenters recommended other measure topics that CMS should consider for the SNF VBP Program including a vaccination measure, specifically the Adult Immunization Status (AIS) measure, as well as measure topics being considered for the SNF QRP, such as depression and pain management. One commenter recommended that CMS consider a measure that assesses SNF residents' access to physical medicine and rehabilitation (PM&R) physicians because the commenter believes that PM&R engagement is important in SNFs where staff may not have the expertise to address medical complications or barriers to therapy participation and progression. Another commenter recommended a measure that evaluates the quality of health benefits being provided to direct care workers. One commenter recommended measures that appropriately incentivize and financially reward high-performing SNFs and identified the Measures Under Consideration (MUC) process as especially important to developing and refining measures.
One commenter recommended that CMS revise the specifications for the Nursing Staff Turnover measure so that the measure only counts gaps in employment of more than 120 days, instead of the current 60 days, as turnover. The commenter expressed that there are many reasons an employee may be on an extended leave of absence for more than 60 days with the intention of returning to work. The commenter believed that the current specifications may unfairly penalize providers and may mislead the public.
One commenter did not support a staffing composite measure because it could reduce the contribution of each staffing metric (Total Nurse Staffing and Nursing Staff Turnover) in assessing a provider's performance.
One commenter recommended that CMS exclude quality measures that are unrelated to the Program's intent. Specifically, the commenter did not support the use of the Total Nurse Staffing and Nursing Staff Turnover measures in the Program because the commenter believed these measures only add reporting and administrative burden for SNFs. Another commenter did not support the inclusion of measures that have not been captured or publicly reported for at least 3 years. This commenter believed that new measures take time for SNFs to understand and establish evidence-based practices for improving performance.
One commenter did not support the use of MDS-based measures in the SNF VBP Program as the commenter believed MDS data are not sufficiently accurate. Another commenter did not support the addition of long stay measures, such as the Falls with Major Injury (Long Stay) and Long Stay Hospitalization measures, because the commenter believed these do not align with the intent of the Program, which is to link Medicare FFS reimbursement with the care and outcomes of Medicare FFS beneficiaries.
Response: We thank the commenters for their continuing feedback. We will take all of this feedback into consideration as we develop future measure-related policies for the SNF VBP Program.
D. SNF VBP Performance Standards
1. Background
We refer readers to the FY 2024 SNF PPS final rule (88 FR 53299 through 53300) for a detailed history of our performance standards policies.
In the FY 2024 SNF PPS final rule (88 FR 53300), we adopted the final numerical values for the FY 2026 performance standards and the final numerical values for the FY 2027 performance standards for the DTC PAC SNF measure.
2. Performance Standards for the FY 2027 Program Year
In the FY 2024 SNF PPS final rule (88 FR 53300), we adopted the final numerical values for the FY 2027 performance standards for the DTC PAC SNF measure, which we provide for SNFs' reference at the bottom of Table 32.
To meet the requirements at section 1888(h)(3)(C) of the Act, we are providing the final numerical performance standards for the remaining measures applicable for the FY 2027 program year: SNFRM, SNF HAI, Total Nurse Staffing, Nursing Staff Turnover, Falls with Major Injury (Long Stay), Long Stay Hospitalization, and DC Function measures. In accordance with our previously finalized methodology for calculating performance standards (81 FR 51996 through 51998), the final numerical values for the FY 2027 program year performance standards are shown in Table 32.
3. Performance Standards for the FY 2028 Program Year
In the FY 2024 SNF PPS final rule (88 FR 53280 through 53281), we finalized that the SNF WS PPR measure will replace the SNFRM beginning with the FY 2028 program year. In that final rule (88 FR 53299 through 53300), we also finalized that the baseline and performance periods for the SNF WS PPR measure will each be 2 consecutive years, and that FY 2025 and FY 2026 is the performance period for the SNF WS PPR measure for the FY 2028 program year.
To meet the requirements at section 1888(h)(3)(C) of the Act, we are providing the final numerical performance standards for the FY 2028 program year for the SNF WS PPR measure as well as the DTC PAC SNF measure. In accordance with our previously finalized methodology for calculating performance standards (81 FR 51996 through 51998), the final numerical values for the FY 2028 program year performance standards for the DTC PAC SNF and SNF WS PPR measures are shown in Table 33.
We note that we will provide the estimated numerical performance standards values for the remaining measures applicable in the FY 2028 program year in the FY 2026 SNF PPS proposed rule.
4. Policy for Incorporating Technical Measure Updates Into Measure Specifications and for Subsequent Updates to SNF VBP Performance Standards Beginning With the FY 2025 Program Year
We are required under section 1888(h)(3) of the Act to establish performance standards for SNF VBP measures for a performance period for a fiscal year. Under that section, we are also required to establish performance standards that include levels of achievement and improvement, the higher of which is used to calculate the SNF performance score, and to announce those performance standards no later than 60 days prior to the beginning of the performance period for the applicable fiscal year. We refer readers to the FY 2017 SNF PPS final rule (81 FR 51995 through 51998) for details on our previously finalized performance standards methodology.
In the FY 2019 SNF PPS final rule (83 FR 39276 through 39277), we finalized a policy that allows us to update the numerical values of the performance standards for a fiscal year if we discover an error in the performance standards calculations. Under this policy, if we discover additional errors with respect to that fiscal year, we will not further update the numerical values for that fiscal year.
We currently calculate performance standards for SNF VBP measures using baseline period data, which are then used, in conjunction with performance period data, to calculate performance scores for SNFs on each measure for the applicable program year. However, during the long interval between the time we finalize the performance standards for the measures and the time that we calculate the achievement and improvement scores for those measures based on actual SNF performance, one or more of the measures may have been technically updated in a way that inhibits our ability to make appropriate comparisons between the baseline and performance period. We believe that to calculate the most accurate achievement and improvement scores for a measure, we should calculate the performance standards, baseline period measure results, and performance period measure results using the same measure specifications.
Therefore, we proposed to adopt a policy that allows us to incorporate technical measure updates into the measure specifications we have adopted for the SNF VBP Program so that these measures remain up-to-date and ensure that we can make fair comparisons between the baseline and performance periods that we adopt under the Program. Further, we proposed that we will incorporate these technical measure updates in a sub-regulatory manner and that we will inform SNFs of any technical measure updates for any measure through postings on our SNF VBP website, listservs, and through other educational outreach efforts to SNFs. These types of technical measure updates do not substantively affect the measure rate calculation methodology. We also recognize that some updates to measures are substantive in nature and may not be appropriate to adopt without further rulemaking. In those instances, we proposed to continue to use rulemaking to adopt substantive updates to SNF VBP measures.
With respect to what constitutes substantive versus non-substantive (technical) measure changes, we proposed to make this determination on a case-by-case basis. Examples of technical measure changes may include, but are not limited to, updates to the case-mix or risk adjustment methodology, changes in exclusion criteria, or updates required to accommodate changes in the content and availability of assessment data. Examples of changes that we might consider to be substantive are those in which the changes are so significant that the measure is no longer the same measure.
We also proposed to expand our performance standards correction policy beginning with the FY 2025 program year such that we will be able to update the numerical values for the performance standards for a measure for a program year if a measure's specifications were technically updated between the time that we published the performance standards for a measure and the time that we calculate SNF performance on that measure at the conclusion of the applicable performance period. Any update we make to the numerical values would be announced via the SNF VBP website, listservs, and through other educational outreach efforts to SNFs. In addition, this policy would have the effect of superseding the performance standards that we establish prior to the start of the performance period for the affected measures, but we stated that we believe them to be necessary to ensure that the performance standards in the SNF VBP Program's scoring calculations enable the fairest comparison of measure performance between the baseline and performance period.
We noted that these proposed policies align with the Technical Updates Policy for Performance Standards that we adopted for the Hospital VBP Program in the FY 2015 IPPS/LTCH PPS final rule (79 FR 50077 through 50079).
Further, we proposed to codify these policies in our regulations. Specifically, we proposed to codify our policy to incorporate technical measure updates into previously finalized SNF VBP measure specifications in a sub-regulatory manner by adding a new paragraph (l)(4) to our regulations at § 413.338. Our current performance standards policies are codified at § 413.338(d)(6) of our regulations. However, we proposed to redesignate that paragraph as new § 413.338(n) of our regulations and to include in paragraph (n) both the existing performance standards policies and this newly proposed expansion of our performance standards correction policy.
We invited public comment on our proposal to adopt a policy for incorporating technical measure updates into the SNF VBP measure specifications and for subsequent updates to the SNF VBP performance standards beginning with the FY 2025 program year. We also invited public comment on our proposal to codify these policies in our regulations.
We received public comments on this proposal. The following is a summary of the comments we received and our responses.
Comment: One commenter supported CMS' proposal to use a sub-regulatory process to incorporate technical measure updates into SNF VBP measure specifications and to update the numerical values for a measure's performance standards if that measure was technically updated between the time we published the performance standards and the time CMS calculates SNF performance on that measure. The commenter further believed that CMS should use notice and comment rulemaking to make substantive measure changes.
Response: We thank the commenter for their support. As stated in the proposed rule (89 FR 23473), we will continue to use rulemaking to adopt substantive updates to SNF VBP measures.
Comment: One commenter supported CMS' proposal to incorporate technical measure updates into the measure specifications adopted for the SNF VBP Program using a sub-regulatory process. However, the commenter recommended that when CMS incorporates technical measure updates for SNF VBP measures outside of regular rulemaking, CMS exclude and suppress the affected measure(s) for all SNFs and base the SNF performance score for the affected program year on the remaining measures.
Response: We thank the commenter for their support of this proposal. With regard to the commenter's recommendation to exclude and suppress SNF VBP measures that have been technically updated, we reiterate that these measure updates are technical in nature and are not anticipated to impact SNF performance significantly. Therefore, we do not see any reason to suppress or exclude these measures from a SNF's performance score. Further, as stated in the proposed rule (89 FR 23473), we would continue to use notice and comment rulemaking to propose and adopt substantive measure updates that significantly affect the measure. These substantive measure updates would be adopted prior to or in conjunction with our announcement of performance standards that reflect the updated measure specifications for the measure for the applicable program year. We would determine whether an update is substantive or non-substantive on a case-by-case basis. Further, we intend to evaluate the impacts of this policy on SNF performance as part of our regular monitoring and evaluation efforts.
Comment: A few commenters did not support CMS' proposal to use a sub-regulatory process to update the numerical values for a measure's performance standards for a program year if that measure's specifications were technically updated between the time we published the performance standards and the time we calculate SNF performance on that measure. The commenters believed that updating previously established performance standards, without proper notice, would limit SNFs' ability to set quality improvement goals and achieve adequate performance, and it would cause confusion among SNFs and consumers because the data are used in more than 1 program year.
Response: We proposed that a measure's specifications may be technically updated between the time we publish the performance standards and the time we calculate the achievement and improvement scores for that measure based on actual SNF performance. We make technical measure updates to measure specifications to ensure the measure scores reflect SNF performance as accurately and completely as possible. However, as stated earlier in this section, since these updates would be technical in nature, they are not anticipated to impact SNF performance significantly. We do not believe that it is fair or appropriate to calculate performance period measure results using the updated measure specifications and then compare those results to the performance standards and baseline period measure results that were calculated using the previous measure specifications to generate the achievement and improvement scores. We view this policy, which allows us to update the numerical values for a measure's performance standards if that measure's specifications were technically updated, as necessary to ensure the accuracy of SNF performance scores, which are based on the performance standards.
We intend to announce updates to the numerical values of the performance standards as soon as we can calculate the updated performance standards after the measure specifications have been technically updated. These announcements would be made via the SNF VBP website, listservs, and through other educational outreach efforts to SNFs. Further, we would not update the performance standards for a measure after the applicable performance period has ended.
We disagree with commenters' suggestion that updating the performance standards for a measure would impact a SNF's ability to set quality improvement goals or their ability to achieve adequate performance. We make technical updates to a measure's specifications to ensure we measure SNF performance as accurately as possible. As stated earlier in this section, we view this policy, which allows us to update the numerical values for a measure's performance standards if that measure's specifications were technically updated, as necessary to ensure that the performance standards in the SNF VBP Program's scoring calculations enable the fairest comparison of measure performance between the baseline and performance period and to ensure the accuracy of SNF performance scores. We also note that while the performance standards we establish under the SNF VBP Program reflect levels of achievement and improvement and are used for the purposes of assessing SNF performance on the measures, they are not intended to be the ceiling for SNF performance on a measure. Therefore, we encourage SNFs to set quality improvements goals that are not limited to the measure rates reflected in the performance standards. With respect to achieving adequate performance, we note that accurate performance standards, which is the goal of this proposed policy, are essential for calculating measure scores and SNF performance scores that reflect the actual provision of care in SNFs.
We also disagree with the commenters' suggestion that this policy would cause confusion because the measure data are used for more than one program year. It is true that measure data are used for more than one program year. For example, the performance period for the DC Function measure for the FY 2027 program year is FY 2025 and the baseline period for the FY 2029 program year is also FY 2025. However, if we make technical updates to a measure's specifications, all future calculations related to that measure will utilize the updated measure specifications. Therefore, we do not believe this would cause confusion among SNFs. We would not be able to update calculations for prior program years because SNFs would have already received their SNF performance scores and payment adjustments. Using the same example as above, if we make technical updates to the measure specifications for the DC Function measure for the FY 2027 program year, we would announce the updated performance standards before the end of the FY 2025 performance period. We would subsequently calculate baseline period results and performance standards for the FY 2029 program year after the end of the FY 2025 baseline period, which would automatically utilize the updated measure specifications.
For our measures with 2-year baseline and performance periods, it may be the case, due to performance periods overlapping, that we need to update the performance standards for more than one program year. If this situation arises, we intend to be as transparent as possible to ensure SNFs have a clear understanding of the impact of the technical measure updates.
In addition, as stated in the proposed rule (89 FR 23474), we intend to announce any updates to the numerical values of the performance standards for affected measures via the SNF VBP website, listservs, and through other outreach efforts to SNFs.
After consideration of public comments, we are finalizing our proposal to incorporate technical measure updates into measure specifications and for subsequent updates to SNF VBP performance standards beginning with the FY 2025 program year. We are also finalizing our proposal to codify these policies in our regulations.
E. SNF VBP Performance Scoring Methodology
1. Background
We refer readers to the FY 2024 SNF PPS final rule (88 FR 53300 through 53304) for a detailed history of our performance scoring methodology. Our performance scoring methodology is codified at § 413.338(d) and (e) of our regulations. We have also codified the Health Equity Adjustment (HEA) at § 413.338(k) of our regulations.
While we did not propose any changes to the previously adopted case minimum requirements, we received one comment. The following is a summary of the comment and our response.
Comment: One commenter expressed concern that the existing case minimum requirements in the SNF VBP Program may reward and penalize random variation, not actual performance, for some providers. The commenter recommended that CMS adopt case minimum requirements that meet a reliability standard of 0.7, which could be accomplished by increasing the minimum case counts to 60. The commenter defined the 0.7 reliability standard as 70 percent of the variation being explained by differences in performance and 30 percent being attributed to random chance. The commenter also suggested extending the performance periods to include multiple years because they believe this will allow more SNFs to meet the higher reliability threshold.
Response: We refer readers to the FY 2023 SNF PPS final rule (87 FR 47585 through 47587) and the FY 2024 SNF PPS final rule (88 FR 53301 through 53302) for the case minimums we have finalized for each of the SNF VBP Program measures. We stated that those case minimums are appropriate for the SNF VBP Program because they ensure the Program requirements only apply to SNFs for which we can calculate reliable measure rates and SNF performance scores. Our testing has also indicated that increasing the case minimum requirements to achieve the reliability standard of 0.7 would result in minimal improvements to a measure's reliability while simultaneously increasing the number of SNFs that would not meet the higher case minimum requirement, which does not align with our goal to ensure as many SNFs as possible can receive a score on a given measure. Therefore, we do not believe it is currently necessary or feasible to adopt case minimum requirements that meet a reliability standard of 0.7.
We also acknowledge the commenter's recommendation to increase measure reliability through longer performance periods and baseline periods and agree this could increase measure reliability. However, as stated in the FY 2016 SNF PPS final rule (80 FR 46422) and the FY 2017 SNF PPS final rule (81 FR 51998 through 51999), we aim to balance measure reliability with recency of data to ensure clear connections between quality measurement and value-based payment. We do not believe that adopting longer performance and baseline periods for all SNF VBP measures appropriately balance these factors. Specifically, longer performance and baseline periods would mean that SNF performance scores and the resulting value-based payments would be based on data further in the past, which is not consistent with our desire to calculate SNF performance scores and value-based payments using as recent as possible measure data.
2. Measure Minimum Policies
a. Background
We refer readers to the FY 2024 SNF PPS final rule (88 FR 53301 through 53303) for details on our previously adopted case minimums and measure minimums. Our case minimum and measure minimum policies are also codified at § 413.338(b) of our regulations. In the proposed rule, we proposed to apply the previously finalized FY 2027 measure minimum to the FY 2028 program year and subsequent years. We did not propose any changes to our previously finalized case minimums.
b. Application of the FY 2027 Measure Minimum to the FY 2028 SNF VBP Program Year and Subsequent Years
In the FY 2024 SNF PPS final rule (88 FR 53301 through 53303), we adopted an updated measure minimum for the FY 2027 program year. Specifically, we finalized that for a SNF to receive a SNF performance score and value-based incentive payment for the FY 2027 program year, SNFs must report the minimum number of cases for four of the eight measures during the applicable performance period. As discussed in the proposed rule, we proposed to apply this measure minimum to the FY 2028 program year and subsequent years, such that SNFs must report the minimum number of cases for at least four measures during the applicable performance period. SNFs that do not meet this measure minimum requirement would be excluded from the applicable program year and receive their adjusted Federal per diem rate for that fiscal year.
Based on our analyses for the FY 2028 program year, which are also applicable to subsequent program years for which we use the same measure set, we estimated that, under this measure minimum, approximately 6 percent of SNFs would be excluded from the Program compared to the approximately 8 percent of SNFs that we estimate would be excluded from the Program in FY 2027. This estimated decrease indicates fewer SNFs would be excluded from the FY 2028 program year than the FY 2027 program year due to the SNF WS PPR measure replacing the SNFRM beginning in FY 2028. We also assessed the consistency of incentive payment multipliers (IPMs), or value-based incentive payment adjustment factors, between FY 2027 and FY 2028 as a proxy for SNF performance score reliability. We found that applying the FY 2027 measure minimum to the FY 2028 program year would have minimal impact on the percentage of SNFs that would receive a net positive IPM and receive a net negative IPM between those 2 fiscal years, which indicates that the reliability of the SNF performance score would be minimally impacted if we applied the FY 2027 measure minimum to the FY 2028 program year. Based on these testing results for FY 2028, we stated that applying the FY 2027 measure minimum to the FY 2028 program year and subsequent years best balances SNF performance score reliability with our desire to ensure that as many SNFs as possible can receive a SNF performance score. We noted in the proposed rule that if we propose in future years to revise the total number of measures in the Program, we would reassess this measure minimum policy to ensure it continues to meet our previously stated goals. If needed, we would propose updates in future rulemaking.
We invited public comment on our proposal to apply the FY 2027 measure minimum to the FY 2028 SNF VBP program year and subsequent program years, such that SNFs must report the minimum number of cases for at least four measures during the applicable performance period.
We received public comments on this proposal. The following is a summary of the comments we received and our responses.
Comment: A few commenters supported the proposed measure minimum for the FY 2028 program year and subsequent years.
Response: We thank the commenters for their support of the measure minimum for FY 2028 program year and subsequent years.
Comment: One commenter did not support the proposed measure minimum and instead recommended that CMS increase the proposed measure minimum to at least six of the eight measures to ensure the program addresses quality in multiple areas.
Response: We disagree with the commenter's recommendation that we adopt a measure minimum of six measures, which the commenter believes would better ensure that the Program addresses quality in multiple areas. As stated in the proposed rule (89 FR 23474), we believe that requiring SNFs to report a minimum of four measures best balances SNF performance score reliability with our desire to ensure that as many SNFs as possible can receive a SNF performance score.
We note that swing bed facilities can report a maximum of four of the eight SNF VBP measures because those facilities do not report Payroll Based Journal (PBJ) data and they do not care for long stay residents, which is defined as stays greater than 100 days. Specifically, subsection 1128I(g) of the Act requires SNFs and NFs to report staffing information based on payroll data. This requirement does not apply to swing bed facilities. Further, the direct care staff in a swing bed facility may not solely provide SNF care and therefore, we do not believe that the payroll (PBJ) data would accurately reflect the staffing levels for providing SNF care only. For this reason, we do not believe that it is fair or appropriate to require swing bed facilities to report PBJ data for the two SNF VBP staffing measures (Total Nurse Staffing and Nursing Staff Turnover measures). In addition, because swing bed facilities do not care for long stay residents, those facilities do not meet the minimum case thresholds to report the Long Stay Hospitalization and Falls with Major Injury (Long Stay) measures. Therefore, if we increased the measure minimum to more than four measures, all swing bed facilities would be excluded from the Program. This does not align with our desire to ensure that as many SNFs as possible are included in the Program and can receive a SNF performance score.
Further, in our testing for the measure minimum of four measures, we found that approximately 60 percent of SNFs would continue to be scored on all eight measures, approximately 87 percent of SNFs would continue to be scored on at least six measures, and as described earlier in this section, over 90 percent will be scored on at least four measures. Therefore, as indicated by our testing of a four measure minimum, the vast majority of SNFs would be included in the Program and would be assessed on their performance across multiple quality areas.
After consideration of public comments, we are finalizing the measure minimum for the FY 2028 program year and subsequent program years as proposed.
3. Potential Next Steps for Health Equity in the SNF VBP Program
In the FY 2024 SNF PPS final rule (88 FR 53304 through 53318), we adopted a Health Equity Adjustment (HEA) that allows SNFs that provide high quality care and care for high proportions of SNF residents who are underserved to earn bonus points. We refer readers to that final rule for an overview of our definition of health equity, current disparities in quality of care in the SNF setting, our commitment to advancing health equity, and the details of the HEA.
In the FY 2024 SNF PPS proposed rule (88 FR 21393 through 21396), we also included a request for information (RFI) entitled “Health Equity Approaches Under Consideration for Future Program Years,” where we noted that significant disparities in quality of care persist in the SNF setting. We stated that the goal of explicitly incorporating health equity-focused components into the Program was to both measure and incentivize equitable care in SNFs. Although the HEA rewards high performing SNFs that care for high proportions of SNF residents with underserved populations, it does not explicitly measure or reward high provider performance among the underserved population. We remain committed to achieving equity in health outcomes for residents by promoting SNF accountability for addressing health disparities, supporting SNFs' quality improvement activities to reduce these disparities, and incentivizing better care for all residents. Through the RFI, we solicited public comment on possible health equity advancement approaches to incorporate into the Program in future program years that could supplement or replace the HEA. We refer readers to the FY 2024 SNF PPS final rule (88 FR 53322) for a summary of the public comments we received in response to the health equity RFI. We are considering these comments as we continue to develop policies, quality measures, and measurement strategies on this important topic.
We are currently exploring the feasibility of proposing future health equity-focused metrics for the Program. Specifically, we are considering different ways of measuring health equity that could be incorporated into the Program as either a new measure, combined to form a composite measure, or as an opportunity for SNFs to earn bonus points on their SNF performance score. These performance metrics described in more detail in the proposed rule would utilize the existing SNF HAI, DC Function, DTC PAC SNF, and SNF WS PPR measures that we previously adopted in the Program. We are considering the development of health-equity-focused versions of these measures because they are either cross-setting or could be implemented in multiple programs. The health-equity focused measures or metrics for bonus points include:
- A high-social risk factor (SRF) measure that utilizes an existing Program measure where the denominator of the measure only includes residents with a given SRF, which would allow for comparisons of care for underserved populations across SNFs;
- A worst-performing group measure that utilizes an existing Program measure and compares the quality of care among residents with and without a given SRF on that measure and places greater weight on the performance of the worst-performing group with the goal of raising the quality floor at every facility; and
- A within-provider difference measure that assesses performance differences between residents (those with and without a given SRF) within a SNF on an existing Program measure, creating a new measure of disparities within SNFs.
We are testing these various measure concepts to determine where current across- and within-provider disparities exist in performance, how we can best incentivize SNFs to improve their quality of care for all residents, including those who may be underserved, and the feasibility of incorporating a health equity-focused measure into the Program.
As we explore these and other options, we are focusing on approaches that:
- Include as many SNFs as possible and are feasible to implement;
- Integrate feedback from interested parties;
- Encourage high quality performance for all SNFs among all residents and discourage low quality performance;
- Are simple enough for SNFs to understand and can be used to guide SNFs in improvement; and
- Meet the goal of incentivizing equitable care to ensure all residents in all SNFs receive high quality care.
We are also exploring how constraints, such as sample size limitations, may impact our ability to effectively incorporate certain approaches into the Program. Lastly, we continue to explore opportunities to align with other CMS quality programs to minimize provider burden.
We received public comments related to potential next steps for health equity in the SNF VBP Program. The following is a summary of the comments we received.
Comment: Several commenters supported incorporating additional health equity components into the SNF VBP Program and offered recommendations for doing so. A few commenters offered recommendations related to health equity-focused measures. Specifically, one commenter recommended a workforce equity metric to incentivize SNFs to promote workforce equity and another commenter encouraged CMS to prioritize the DC Function and DTC PAC SNF measures when assessing for different performance outcomes based on the existence of social determinants of health. One commenter requested that CMS not create additional burden when developing health equity-focused measures and instead utilize existing claims or MDS data. One commenter recommended that CMS consider and incorporate feedback from interested parties, such as nurses and other providers, when developing possible health equity-focused measures. Another commenter encouraged CMS to work with the CBE to develop meaningful health equity-focused measures.
A few commenters recommended that CMS consider utilizing proxies other than DES for defining the underserved population. One commenter recommended that CMS assess the impact of health equity measures in non-SNF settings and develop a methodology that can be applied across multiple care settings. Another commenter suggested that CMS should require all SNFs to submit data on health equity to be eligible for SNF VBP incentive payments. Lastly, one commenter recommended that CMS offer education and resources that help SNFs learn how health equity impacts their population and how to make changes and develop interventions based on that information.
Response: We thank commenters for their recommendations. We will take these into consideration as we continue our work on developing the best approaches for incorporating health equity into the Program.
F. Updates to the SNF VBP Review and Correction Process
1. Background
We refer readers to the FY 2024 SNF PPS final rule (88 FR 53325 through 53326) and to § 413.338(f) of our regulations for details on the SNF VBP Program's public reporting requirements and the two-phase review and correction process that we have adopted for the Program. We also refer readers to the SNF VBP website ( https://www.cms.gov/medicare/quality/nursing-home-improvement/value-based-purchasing/confidential-feedback-reporting-review-and-corrections) for additional details on our review and correction process. In Phase One of the review and correction process, we accept corrections for 30 days after distributing the following quarterly confidential feedback reports to SNFs: the two Full-Year Workbooks (one each for the baseline period and performance period), generally released in December and June, respectively. Corrections are limited to errors made by CMS or its contractors when calculating a measure rate. In the FY 2022 SNF PPS final rule (86 FR 42516 through 42517), we finalized that SNFs are not able to correct any of the underlying administrative claims data used to calculate a SNF's readmission measure rate during Phase One of the SNF VBP review and correction process. For corrections to the underlying administrative claims data to be reflected in the SNF VBP Program's quarterly confidential feedback reports, the SNF must submit the claims correction request to their MAC and the MAC must process the correction before the “snapshot date.” For the SNFRM, the quarterly confidential feedback reports will not reflect any claims corrections processed after the date of the claims snapshot, which is 3 months following the last index SNF admission in the applicable baseline period or performance period.
In Phase Two of the review and correction process, SNFs may submit corrections to SNF performance scores and rankings only. We accept Phase Two corrections for 30 days after distributing the Performance Score Report that we generally release in August of each year.
Under our current review and correction policy, the SNF must identify the error for which it is requesting correction, explain its reason for requesting the correction, and submit documentation or other evidence, if available, supporting the request. SNFs must submit correction requests to the SNF VBP Program Help Desk, which is currently available at SNFVBP@rti.org, and the requests must contain:
- The SNF's CMS Certification Number (CCN),
- The SNF's name,
- The correction requested, and
- The reason for requesting the correction, including any available evidence to support the request.
For all review and correction requests, we will review the requests and notify the requesting SNF of the final decision. We will also implement any approved corrections before the affected data becomes publicly available.
In the FY 2025 SNF PPS proposed rule (89 FR 23476), we proposed to apply our existing Phase One review and correction process to all measures adopted in the Program regardless of the data source for a particular measure. We also proposed “snapshot dates” for the new SNF VBP measures and to codify those snapshot dates at revised § 413.338(f)(1). We also proposed to redesignate current § 413.338(f)(1) as § 413.338(f)(2) and to revise that paragraph to state that the underlying data used to calculate measure rates cannot be corrected by SNFs during the SNF VBP review and correction process.
We received comments on our review and correction proposals. The following is a summary of the comments we received and our responses.
Comment: Several commenters expressed support for CMS' proposal to apply the existing review and correction policies to additional measure types.
Response: We thank the commenters for their support.
Comment: A few commenters recommended that CMS make additional allowances in the review and correction process for SNFs. Specifically, one commenter suggested that CMS extend the “snapshot dates” to ensure that SNFs have adequate time to report accurate measure data. Another commenter suggested that CMS adopt a waiver policy for data errors that fall outside the “snapshot dates” that would allow SNFs to incorporate corrections into their performance data provided that the SNF otherwise complied with reporting deadlines.
Response: We thank the commenters for these suggestions. In general, we adopt “snapshot dates” for the purposes of review and correction so we can ensure that we have as much complete and accurate data as possible to calculate measure scores and performance scores. We proposed to calculate the measure rates using a static “snapshot” of data accessed on a specific date. The use of a data “snapshot” enables us to provide as timely quality data as possible, both to SNFs for the purpose of quality improvement, and to the public for the purpose of transparency. After the data “snapshot” is taken through our extraction of Medicare claims data, PBJ staffing data, or MDS assessment data, it takes several months to incorporate other data needed for the measure calculations, generate and check the calculations, as well as program, populate, and deliver the confidential quarterly reports and accompanying data to SNFs. Because several months lead-time is necessary after acquiring the input data to generate these calculations, if we were to delay our data extraction point beyond the proposed measure snapshot dates, we believe this would create an unacceptably long delay both for SNFs to receive timely data for quality improvement and transparency, and incentive payments for purposes of this Program. For the SNFRM and other claims-based measures, we believe that a 3-month claims “run-out” period is a reasonable period that allows SNFs time to correct their administrative claims or add any missing claims before those claims are used for measure calculation purposes while enabling us to timely calculate the measure. For PBJ staffing data and MDS assessment data, the snapshot date aligns with the timeline to which SNFs already adhere for corrections to their data within the Nursing Home Quality Improvement Program and SNF QRP, respectively. We believe this proposed policy would address both fairness and operational concerns associated with calculating measure rates and would provide consistency across value-based purchasing programs. We understand that these “snapshot dates” may occasionally require SNFs to work quickly to review their performance data, but we believe that these deadlines are necessary to ensure that the scoring and payment calculations that we make are as accurate as possible while also meeting our statutory deadlines.
2. Application of the Existing Phase One Review and Correction Policy to All Claims-Based Measures Beginning With the FY 2026 Program Year and “Snapshot Dates” for Recently Adopted SNF VBP Claims-Based Measures
In the FY 2023 SNF PPS final rule, we adopted the SNF HAI measure beginning with the FY 2026 SNF VBP program year (87 FR 47564 through 47570), and the DTC PAC SNF measure beginning with the FY 2027 SNF VBP program year (87 FR 47576 through 47580). In the FY 2024 SNF PPS final rule, we adopted the Long Stay Hospitalization measure beginning with the FY 2027 SNF VBP program year (88 FR 53293 through 53296), as well as the SNF WS PPR measure beginning with the FY 2028 SNF VBP program year (88 FR 53277 through 53280). Each of these measures is calculated using claims data.
We proposed to apply our existing Phase One review and correction process to all SNF VBP Program measures calculated using claims data. That is, Phase One corrections for claims-based measures would be limited to errors made by CMS or its contractors when calculating the measure rates. For corrections to the underlying administrative claims data to be reflected in the SNF VBP Program's quarterly confidential feedback reports, the SNF must submit any claims correction requests to their MAC before the “snapshot date” to ensure that those corrections are reflected fully in measure calculations. Any corrections made to claims following the “snapshot date” would not be reflected in our subsequent scoring calculations.
For the SNF HAI, DTC PAC SNF, and SNF WS PPR measures, we proposed to define the “snapshot date” as 3 months following the last SNF discharge in the applicable baseline period or performance period to align with the “snapshot date” we previously adopted for the SNFRM. We refer readers to the FY 2022 SNF PPS final rule (86 FR 42516 through 42517) where we explain our rationale for selecting 3 months as the “snapshot date.”
For the Long Stay Hospitalization measure, we proposed to define the “snapshot date” as 3 months following the final quarter of the applicable baseline period or performance period. For example, for the FY 2027 SNF VBP program year, the performance period is FY 2025. The final quarter of the performance period is July 1 through September 30, 2025. The “snapshot date” for this performance period is December 31, 2025.
We invited public comment on our proposal to apply our existing Phase One review and correction process to all SNF VBP claims-based measures and to adopt “snapshot dates” for recently adopted SNF VBP claims-based measures.
We received public comments on these proposals. The following is a summary of the comments we received and our response.
Comment: A few commenters supported CMS' proposal to define the “snapshot date” for the Long Stay Hospitalization measure as the 3 months following the final quarter of the applicable baseline period or performance period. One commenter noted that the proposed “snapshot date” is consistent with the “snapshot dates” CMS previously adopted for other claims-based measures, such as the SNFRM. Another commenter agreed that three months should be sufficient for SNFs to identify HAIs that may need to be corrected for the SNF HAI measure and therefore supported our proposal to align its time period with previously adopted “snapshot dates”.
Response: We thank the commenters for their support. We agree that this “snapshot date” is consistent with other “snapshot dates” CMS has previously adopted. In the FY 2022 SNF PPS final rule (86 FR 42516 through 42517), we noted that since several months of lead-time is necessary after acquiring the input data to generate the SNFRM calculations, if we were to delay our data extraction point beyond the proposed measure “snapshot date”, we believed this would create an unacceptably long delay both for SNFs to receive timely data for quality improvement and transparency, and incentive payments for purposes of this program. We believe that this rationale for the SNFRM also applies to the additional SNF VBP claims-based measures. We believe that a 3-month claims “run-out” period allows SNFs time to correct their administrative claims or add any missing claims before those claims are used for measure calculation purposes, while enabling us to timely calculate the measure.
After consideration of public comments, we are finalizing these policies as proposed.
3. Application of the Existing Phase One Review and Correction Policy to PBJ-Based Measures Beginning With the FY 2026 Program Year and “Snapshot Dates” for SNF VBP PBJ-Based Measures
In the FY 2023 SNF PPS final rule (87 FR 47570 through 47576), we adopted the Total Nurse Staffing measure beginning with the FY 2026 SNF VBP program year. Additionally, in the FY 2024 SNF PPS final rule (88 FR 53281 through 53286), we adopted the Nursing Staff Turnover measure beginning with the FY 2026 SNF VBP program year. Each of these measures is calculated using electronic staffing data submitted by each SNF for each quarter through the Payroll Based Journal (PBJ) system, along with daily resident census information derived from MDS 3.0 standardized patient assessments in the case of the Total Nurse Staffing measure.
We proposed to apply our existing Phase One review and correction process to SNF VBP Program measures calculated using PBJ staffing data. That is, Phase One corrections would be limited to errors made by CMS or its contractors when calculating the measure rates for the PBJ-based measures applicable in the SNF VBP Program. For corrections to the underlying PBJ data to be reflected in the SNF VBP Program's quarterly confidential feedback reports, the SNF must make any corrections to the underlying data within the PBJ system before the “snapshot date.” Any corrections made to PBJ staffing data following the “snapshot date” would not be reflected in our subsequent scoring calculations.
For measures calculated using PBJ staffing data, we proposed to define the “snapshot date” as 45 calendar days after the last day in each fiscal quarter. This deadline is consistent with the CMS Nursing Home Quality Improvement deadline, which requires that PBJ data submissions must be received by the end of the 45th calendar day (11:59 p.m. Eastern Time (ET)) after the last day in each fiscal quarter to be considered timely. We aim to align CMS quality programs to the extent possible to reduce confusion and burden on providers. For more information about submitting staffing data through the PBJ system, we refer readers to the CMS Staffing Data Submission web page at https://www.cms.gov/medicare/quality/nursing-home-improvement/staffing-data-submission.
We invited public comment on our proposal to apply our existing Phase One review and correction process to SNF VBP PBJ-based measures and to adopt “snapshot dates” for SNF VBP PBJ-based measures.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: One commenter recommended that CMS adopt a “snapshot date” for PBJ-based measures that allows PBJ staffing data corrections for up to 3 months after the end of the applicable baseline period or performance period. The commenter believed that this “snapshot date” would provide consistency with the claims-based measures. The commenter also suggested that, if CMS considers claims-based measures as the gold standard of measurement, then CMS should treat other types of measures similarly where possible.
Response: We thank the commenter for this feedback. However, as we noted in the proposed rule (89 FR 23476), we proposed the “snapshot date” for PBJ data as 45 calendar days after the last day in each fiscal quarter to align with the CMS Nursing Home Quality Improvement deadline. For the Nursing Home Quality Improvement Program, data submissions must be received in PBJ by the end of the 45th calendar day after the last day in each fiscal quarter to be considered timely. If the SNF VBP Program were to allow corrections to this data past this date as the commenter suggests, it could result in different reported measure rates for the SNF VBP program and the Nursing Home Quality Improvement for the same measures. This could result in confusion from SNFs and the public when these data are publicly reported.
Comment: One commenter recommended that CMS provide SNFs a preview report (like the 1705D PBJ Staffing Data Report) after the final submission is complete for the quarter. The commenter further suggested that facilities should be provided at least 15 days after this point to review and correct the submitted PBJ data. The commenter explained that, if a facility uses a vendor to submit data on their behalf, the facility is held responsible for errors even if those errors were made by the vendor and were outside of the SNF's control. In addition, the commenter stated that there may be unexpected circumstances where there are errors or missed information identified by the facility later despite the facility's good faith efforts to submit PBJ data accurately and in a timely manner. The commenter noted that this additional time is important for PBJ-based measures, as the recently developed Nursing Staff Turnover measure requires 6 consecutive months of PBJ data and if any quarter of data is missing or unusable, the staff turnover rates may not be calculated or may be flawed, leaving consumers without information on a facility's true performance.
Response: We will consider whether it would be feasible to provide SNFs with preview reports in addition to the quarterly confidential feedback reports that we provide to SNFs under section 1888(g) and the SNF performance score reports that we provide to notify SNFs of their performance scores and incentive payment percentages. However, we note that we proposed the 45-day “snapshot date” for PBJ data to align with the CMS Nursing Home Quality Improvement deadline, and we continue to believe that this alignment will help SNFs comply with measure and data requirements across CMS quality programs. While the PBJ data is used for multiple measures across CMS quality programs, SNFs are required to submit the direct care staffing information in one centralized location via the PBJ.
Further, we believe that SNFs must work closely with any vendors with which they operate to ensure that data submissions are fully accurate before they are provided to CMS.
After consideration of public comments, we are finalizing these policies as proposed.
4. Application of the Existing Phase One Review and Correction Policy to MDS-Based Measures Beginning With the FY 2027 Program Year and “Snapshot Dates” for SNF VBP MDS-Based Measures
In the FY 2024 SNF PPS final rule (88 FR 53286 through 53293), we adopted the Falls with Major Injury (Long Stay) and DC Function measures, both beginning with the FY 2027 SNF VBP program year. These two measures are calculated using data reported by SNFs on the MDS 3.0.
We proposed to apply our existing Phase One review and correction process to SNF VBP Program measures calculated using MDS data. That is, Phase One corrections would be limited to errors made by CMS or its contractors when calculating the measure rates for the MDS-based measures applicable in the SNF VBP Program. For corrections to the underlying MDS data to be reflected in the SNF VBP Program's quarterly confidential feedback reports, the SNF must make any corrections to the underlying data via the internet Quality Improvement Evaluation System (iQIES) before the “snapshot date.” Any corrections made to the MDS data following the “snapshot date” would not be reflected in our subsequent scoring calculations.
For the DC Function and Falls with Major Injury (Long Stay) measures, we proposed that the “snapshot date” is the February 15th that is 4.5 months after the last day of the applicable baseline or performance period. However, if February 15th falls on a Friday, weekend, or Federal holiday, the data submission deadline is delayed until 11:59 p.m. ET on the next business day. For example, for the FY 2027 SNF VBP program year, the performance period is FY 2025 (October 1, 2024, through September 30, 2025). The “snapshot date” for this performance period would normally be February 15, 2026. However, because February 15, 2026, falls on a Sunday, the snapshot date would be extended until the next business day, which is Tuesday, February 17, 2026, due to Monday, February 16, 2026, being a Federal holiday. This is consistent with the SNF QRP QM User's Manual available at https://www.cms.gov/files/document/snf-qm-calculations-and-reporting-users-manual-v50.pdf-0.
We invited public comment on our proposal to apply our existing Phase One review and correction process to SNF VBP MDS-based measures and to adopt “snapshot dates” for SNF VBP MDS-based measures.
We received one public comment on these proposals. The following is a summary of the comment we received and our response.
Comment: One commenter supported CMS' proposal to define the “snapshot date” for MDS-based measures as 4.5 months after the last day of the applicable baseline or performance period, noting that this timeline closely aligns with deadlines for claims-based measures.
Response: We thank the commenter for their support.
After consideration of public comments, we are finalizing these policies as proposed.
G. Updates to the SNF VBP Extraordinary Circumstances Exception Policy
1. Background
Our Extraordinary Circumstances Exception (ECE) policy, which allows SNFs to request an exception to the SNF VBP requirements for one or more calendar months when there are certain extraordinary circumstances beyond the control of the SNF, is currently codified at § 413.338(d)(4) of our regulations. We proposed to redesignate that paragraph as new § 413.338(m) of our regulations to ensure the policy remains effective beyond FY 2025. We also proposed to amend our existing ECE policy to include the proposed changes discussed later in this section, as well as to make other technical updates to enhance the clarity of the ECE policy in our regulations.
2. Expanding the Reasons a SNF May Submit an Extraordinary Circumstance Exception Request Beginning With the FY 2025 Program Year
Section 413.338(d)(4)(ii) of our regulations currently states that a SNF may request an ECE if the SNF is able to demonstrate that an extraordinary circumstance affected the care provided to its residents and subsequent measure performance. We proposed to expand this policy to also allow a SNF to request an ECE if the SNF can demonstrate that, because of the extraordinary circumstance, it cannot report SNF VBP data on one or more measures by the specified deadline. This expanded policy would avoid penalizing SNFs due to circumstances out of their control and would also align the SNF VBP ECE policy with the ECE policies we have adopted for the SNF QRP and Home Health QRP.
If we grant an ECE to a SNF under the SNF VBP, we would, as previously finalized, calculate a SNF performance score that does not include the SNF's performance on the measure or measures during the months the SNF was affected by the extraordinary circumstance.
We discuss the comments we received on this proposal and our responses in the next section.
3. Updates to the Instructions for Requesting an Extraordinary Circumstance Exception Beginning With the FY 2025 Program Year
Under our current ECE policy, when a SNF requests an ECE, the SNF must complete an Extraordinary Circumstances Request form (available on https://qualitynet.cms.gov) and send the form, along with supporting documentation, to the SNF VBP Program Help Desk within 90 days of the date that the extraordinary circumstance occurred.
The most recent version of the ECE Request Form no longer includes information related to the SNF VBP Program. Although the previous form is still available, once it is replaced with the new version, SNFs will no longer be able to use this new version of the form when submitting an ECE request for the SNF VBP Program. Accordingly, we proposed to update our policy to align with the current SNF QRP ECE request submission process, which does not require the completion of a form and instead requires SNFs to submit specific information via email to a Help Desk. We proposed that, beginning with the FY 2025 program year, a SNF may request an ECE by sending an email with the subject line “SNF VBP Extraordinary Circumstances Exception Request” to the SNF VBP Program Help Desk with the following information:
- The SNF's CMS Certification Number (CCN);
- The SNF's business name and business address;
- Contact information for the SNF's chief executive officer (CEO) or CEO-designated personnel, including all applicable names, email addresses, telephone numbers, and the SNF's physical mailing address (not a P.O. Box);
- A description of the event, including the dates and duration of the extraordinary circumstance;
- Available evidence of the impact of the extraordinary circumstance on the care the SNF provided to its residents or the SNF's ability to report SNF VBP measure data, including, but not limited to, photographs, media articles, and any other materials that would aid CMS in determining whether to grant the ECE;
- A date when the SNF believes it will again be able to fully comply with the SNF VBP Program's requirements and a justification for the proposed date.
We invited public comment on our proposals to expand the reasons a SNF may request an extraordinary circumstances exception, to update the instructions for requesting an extraordinary circumstances exception under the SNF VBP Program, and to codify this expanded ECE policy in our regulations.
We received public comments on these proposals. The following is a summary of the comments we received and our responses.
Comment: A few commenters supported CMS' proposal to expand the ECE policy to allow SNFs to request an ECE if the SNF can demonstrate that, as a result of an extraordinary circumstance, the SNF cannot report SNF VBP data on one or more measures by the specified deadline.
Response: We thank the commenters for their support. As we stated in the proposed rule, we believe this policy will avoid penalizing SNFs due to circumstances out of their control.
Comment: One commenter supported CMS' proposal to amend the existing regulation text for the ECE policy so that the policy remains in place past FY 2025.
Response: We thank the commenter for their support of this proposal.
Comment: A few commenters supported CMS' proposal to update the instructions for requesting an ECE because it will align the SNF VBP process with the existing process used by the SNF QRP. One commenter believed that eliminating the requirement to submit the distinct ECE form will be effective and efficient.
Response: We thank the commenters for their support. We agree that these updates will streamline the process and enhance alignment with the SNF QRP process for requesting an ECE.
Comment: A few commenters recommended that CMS align and streamline the process for submitting and receiving an ECE across programs, such as the SNF VBP Program and SNF QRP, so that SNFs can easily request an ECE. One commenter specifically recommended further streamlining the process for submitting an ECE request so that if a SNF is granted an ECE by CMS for another program, that ECE is automatically applied to the SNF VBP Program. Another commenter recommended that CMS provide clear information regarding the ECE request processes.
Response: We thank the commenters for their recommendations. We will consider ways to further streamline the ECE process in future rulemaking. We also intend to work to ensure that information related to ECE request processes is accessible to providers. We note that the current instructions for requesting an ECE are available on the SNF VBP website (available at: https://www.cms.gov/medicare/quality/nursing-home-improvement/value-based-purchasing/extraordinary-circumstance-exception). We will update those instructions to include the changes that we are finalizing in this final rule. Along with providing the new ECE instructions on the SNF VBP website, we will consider additional channels of communication that we can leverage to introduce the new ECE request instructions and to clarify any details. Potential methods include, but are not limited to Listservs, Open Door Forums, Listening sessions and webinars, and the CMS News Bulletin. Furthermore, the SNF VBP Program Help Desk, which is currently available at SNFVBP@rti.org, will be accessible to SNFs who are seeking support for the new ECE request instructions or have any questions regarding them.
After consideration of public comments, we are finalizing our proposals to expand the reasons a SNF may request an extraordinary circumstances exception and to update the instructions for requesting an extraordinary circumstances exception under the SNF VBP Program as proposed. We are also finalizing our proposal to codify this expanded ECE policy in our regulations.
IX. Nursing Home Enforcement
A. Background
The Biden-Harris Administration is committed to ensuring that all residents living in nursing homes receive safe, high-quality care. This includes making certain that all Americans, including older Americans and people with disabilities, live in a society that is accessible, inclusive, and equitable. To ensure that residents are receiving high-quality, and safe care, Long-Term Care (LTC) facilities that participate in the Medicare and/or Medicaid program, must be certified as meeting Federal participation requirements. LTC facilities are certified as a skilled nursing facility (SNF) in Medicare and a nursing facility (NF) in Medicaid, or a dually-certified SNF/NF in both programs, as specified in sections 1819 and 1919 of the Social Security Act (Act), respectively, and in regulations at 42 CFR part 483, subpart B.
Section 1864(a) of the Act authorizes the Secretary to enter into agreements with State Survey Agencies (SSAs) to conduct surveys (that is, inspections) to determine whether SNFs and NFs meet the Federal participation requirements for Medicare (generally referred to as requirements or Conditions of Participation (CoPs)). Section 1902(a)(33)(B) of the Act provides for SSAs to perform the same survey tasks for facilities participating or seeking to participate in the Medicaid program. See also, section 1919(g) of the Act. The results of these surveys are used by CMS and the State Medicaid agency, respectively, as the basis for a decision to enter into, deny, or terminate a provider agreement with the facility. They are also used to determine whether one or more enforcement remedies should be imposed when noncompliance with requirements is identified. Sections 1819(h) and 1919(h) of the Act. Surveyors observe the provision of care and services to residents, conduct interviews, and review facility and residents' documentation to determine compliance with Federal requirements and ensure the residents' health and safety are adequately protected.
Under sections 1819(f)(1) and 1919(f)(1) of the Act, the Secretary must ensure that the enforcement of compliance with the participation requirements is adequate to protect the health, safety, welfare, and rights of the residents and to promote the effective use of public money. Additionally, under sections 1819(h)(2)(B) and 1919(h)(3)(C) of the Act, criteria must be specified as to when and how enforcement remedies are applied, the amounts of fines, and the severity of each remedy imposed. Criteria must also be designed to minimize the time between the identification of violations and the final imposition of the remedies. Under sections 1819(h)(2)(B) and 1919(h)(3)(C) of the Act, civil money penalties (CMPs) are one of the Federal statutory enforcement remedies available to the Secretary and the States to address facility noncompliance with the requirements. Under sections 1819(h)(2)(B)(ii)(I) and 1919(h)(3)(C)(ii)(I) of the Act, CMPs may be imposed to remedy noncompliance at amounts not to exceed $10,000 for each day of noncompliance (as annually adjusted by inflation by the Federal Civil Penalties Inflation Adjustment Act Improvements Act of 2015 (the 2015 Act). The statute also permits the Secretary and the States to impose a CMP for each day of noncompliance, even if a facility has since returned to substantial compliance as documented by an intervening standard survey (sections 1819(h)(2)(A) and 1919(h)(1) and (3) of the Act providing that if a facility is found to be in compliance with the requirements, “. . . but, as of a previous period, did not meet such requirements, [the Secretary provide for] a civil money penalty . . . for the days in which he finds that the facility was not in compliance with such requirements”). The Secretary must follow the procedures set out in section 1128A of the Act in processing these CMP remedies. (Sections 1819(h)(2)(B)(ii)(I) and 1919(h)(3)(C)(ii)(I) of the Act)
The regulations that govern the imposition of CMPs and other remedies authorized by the statute were published on November 10, 1994 (59 FR 56116), and subsequently revised on September 28, 1995 (60 FR 50118), March 18, 1999 (64 FR 13354 through 13360), March 18, 2011 (76 FR 15106), and September 6, 2016 (81 FR 61538). The nursing home enforcement rules are set forth in 42 CFR part 488, subpart F, and the provisions directly affecting CMPs imposed for noncompliance with the requirements are set forth in §§ 488.430 through 488.444. In general, an enforcement action imposed is based on the severity of harm or potential for more than minimal harm to residents that results and the scope of how many residents were affected by the cited noncompliance. This is intended to ensure prompt and sustained compliance for the future, incentivizing the facility to take appropriate actions to permanently correct their noncompliance and protect residents' health and safety in the future. For example, if residents experienced serious harm due to noncompliance (including death), a less impactful enforcement remedy may not compel the facility to take the appropriate actions to correct and prevent a similar event from occurring in the future, leaving residents at risk for serious harm, injury, or death.
Under 42 CFR 488.438, the amount of CMPs increases based on the severity and/or extent of the harm or potential for more than minimal harm that might result from noncompliance. Current regulations at § 488.408 allow for penalties to be assessed in the upper range of $3,050 to $10,000 per day (PD) or $1,000 to $10,000 per instance (PI), as annually adjusted for inflation for noncompliance that constitutes immediate jeopardy (IJ) to resident health and safety, while penalties in the lower range of $50 to $3,000 PD or $1,000 to $10,000 PI of noncompliance, as annually adjusted for inflation, may be imposed where immediate jeopardy does not exist.
Under the current regulations, the State and/or CMS must decide whether to select either a PD or PI CMP when considering whether a CMP will be used as a remedy. A PD CMP is an amount that may be imposed for each day a facility is not in compliance until the facility corrects the noncompliance and achieves substantial compliance. A PI CMP is an amount imposed for each instance in which a facility is not in substantial compliance. The current enforcement regulations at 42 CFR part 488, subpart F, do not authorize the use of both types of CMPs during the same survey, nor do they allow for multiple PI CMPs to be imposed for multiple instances within the same noncompliance deficiency that occurred on different days during a survey.
While there is no statutory limitation of both a PI and PD being imposed on the same survey, we specified in the rulemaking that revised § 488.430(a) (published on March 18, 1999 (64 FR 13360)), that we would not impose both PD and PI CMPs during a survey. Instead, the 1999 rule required that “a concomitant decision must be made whether the civil money penalty will be based on a determination of per instance or per day” (64 FR 13356). Additionally, we noted that an “instance” means a singular event of noncompliance or single deficiency under a distinct regulatory area identified by an administrative “F tag” number used as reference on the CMS-2567, Statement of Deficiencies. ( Id.) We proposed revisions to this limitation to enable more types of CMPs to be imposed during a survey once a CMP remedy is selected, within the statutory and regulatory limits, allowing penalties to be better aligned with the noncompliance identified during the survey and for more consistency of CMP amount across the nation. PI CMPs are often imposed in certain circumstances, such as when noncompliance existed but was corrected prior to the survey and for isolated instances of noncompliance unrelated to resident abuse. PI CMPs may also be imposed in cases where a deficiency is found, but the facility has not had any citations of actual or serious harm on any survey in the past three years. A PI CMP has typically not been imposed for findings of abuse or neglect, when there is continued noncompliance, or when the facility has a history of the same type of noncompliance causing actual harm to residents. PD CMPs, however, are generally imposed when these scenarios do not exist, and the facility has a history of similar noncompliance. For example, if a facility was found to be out of compliance with the requirements to prevent accidents where a resident was injured during a transfer from a wheelchair to the bed, and this was cited as an isolated instance of noncompliance that caused actual harm to a resident, a PI CMP may be imposed. We developed a Civil Money Penalty Analytic Tool to help determine CMP amounts when a CMP is one of the selected remedies, per sections 1819(h)(2)(B)(ii) and 1919(h)(3)(C)(ii) of the Act; 42 CFR 488.404 and 488.438.
The Biden-Harris Administration is committed to ensuring that all residents living in Medicare and Medicaid nursing homes receive safe, high-quality care. Specifically, in February 2022, alongside a suite of other reforms, CMS committed to expanding financial penalties and other enforcement remedies to improve the safety and quality of care in the Nation's certified nursing homes.[106] As part of this effort, CMS examined the use of PD and PI CMPs and CMP impositions across states from January 1, 2022, to December 31, 2022. Based on this analysis, CMS believes that the prior approach regarding CMPs was not as effective as desired to improve patient safety. We found national variations in the length of time PD CMPs are imposed based on when the noncompliance occurred, when the survey was performed, and when the facility was found to have corrected the noncompliance. For example, from January 1, 2022, to December 31, 2022, the State with the shortest average number of days for PD CMP imposition was 1 day, and the longest average number of days in a State was 43 days. This results in vastly differing PD CMP amounts across the States based on the number of days of noncompliance, as well as the date the survey was conducted, rather than being more focused on the potential or actual harm that a deficiency may cause to residents. In other words, the same type of noncompliance could exist in two facilities in different states, but the PD CMP amounts would be different simply due to the number of days between the identification of noncompliance by the surveyor and the date of correction by the facility. We believe that this results in at least two problems. First, it could create a perception of inequity in the total amount calculated for a CMP. Second, it prevents us from holding some facilities responsible for failing to adequately protect residents' health, safety, and well-being. Take, for example, a survey that finds noncompliance with the requirements of participation that increases the likelihood of serious injury, harm, impairment, or death to residents—such as when residents are susceptible to falls while not being monitored (even when no resident actually fell as a result of the failure to monitor). If this deficiency is identified to have started 100 days prior to the survey, a PD CMP would accrue for each of the 100 days and each additional day until the facility corrected its noncompliance, resulting in a very high CMP. Conversely, another facility's similar noncompliance might result in serious harm to a resident, such as when two residents fall due to failures to monitor, resulting in serious injury. However, if these falls are identified to have occurred one and two days prior to the survey, a PD CMP would only accrue for 2 days and each additional day until the noncompliance was corrected, resulting in a relatively low CMP that may not encourage prompt or lasting compliance.
These scenarios show how the timing of a survey can potentially result in a higher CMP for similar noncompliance that resulted in less harm to residents. As such, we want to ensure that CMS retains the authority to impose CMPs related to the nature of the harm that is caused by—or could be caused by—a facility's noncompliance and the length of such noncompliance, rather than the date that a standard survey was conducted or a finding of noncompliance was identified, even if the administration of imposing the CMP occurs after another survey has been conducted. This approach can help prevent noncompliance from occurring writ large, rather than just addressing it once identified.
Therefore, as discussed in the proposed rule, we proposed to expand and strengthen our enforcement process by revising the regulations to increase CMS's flexibility when a CMP is the selected remedy and allow for multiple PI CMPs to be imposed for the same type of noncompliance, allow for both PD and PI CMPs to be imposed for noncompliance findings in the same survey, as well as ensure that the amount of a CMP does not depend solely on the date that the most recent standard survey is conducted or the date that surveyors identified a finding of noncompliance. With these revisions, in certain circumstances, CMS or the State may use the survey start date when imposing a PD CMP instead of the beginning date of the noncompliance, which maintains the benefit of CMPs accruing to incentivize swift correction to protect existing residents' safety and continuous compliance to protect future residents' safety. In other words, by creating the ability to impose a PI CMP and PD CMP on the same survey, CMS or the State could impose a PI CMP to address the noncompliance that occurred in the past or prior to the survey, and a PD CMP beginning at the start of the survey and continuing until the facility has corrected its noncompliance. Additionally, if multiple instances of noncompliance occurred prior to the survey, CMS or the State could impose multiple PI CMPs, as well as a PD CMP. This helps ensure that similar types of noncompliance receive similar CMPs regardless of how many days prior to the survey it occurred and ensures facilities are motivated to correct their noncompliance as soon as possible after the surveyors identify it.
These revisions are not intended to expand the type of deficiencies that are subject to PD and PI CMPs. The States and CMS would continue to follow the existing criteria for imposing a PD CMP or PI CMP, including imposing a PD or PI CMP for noncompliance that occurred prior to the start of a survey. Rather, these revisions would allow for more consistent CMP amounts imposed across the nation and would expand the current enforcement to allow for CMPs that more closely align with the noncompliance that occurred. These actions will help to better ensure that compliance is quickly achieved and is lasting to ensure resident safety.
In the April 3, 2024, Federal Register (89 FR 23424), we published the proposed rule setting forth our proposal for revising the requirements for imposing CMPs. In the proposed rule, we stated that our goal is to enable CMS and the States to impose CMPs to better reflect the type of noncompliance that occurred.
1. Imposing Multiple Per Instance Civil Money Penalties for the Same Type of Noncompliance
We proposed at § 488.408(e)(2)(ii), that for each instance of noncompliance, CMS and the State may impose a PD CMP of $3,050 to $10,000 (as adjusted under 45 CFR part 102), a PI CMP of $1,000 to $10,000 (adjusted under 45 CFR part 102), or both, in addition to the remedies specified in § 488.408(e)(2)(i).
2. Imposing Per Instance and Per Day Civil Money Penalties on the Same Survey
We proposed at §§ 488.408(e)(2)(ii) and 488.430(a) to expand our authority to impose both a PI CMP and a PD CMP, not to exceed the statutory and regulatory maximum amount on any given day, even when combined, when surveyors identify noncompliance.
3. Timing of Enforcement
We proposed at § 488.430(b) to allow the imposition of CMPs for noncompliance that was identified since the last three standard surveys.
B. Provisions of the Proposed Regulations
1. Imposing Multiple Per Instance Civil Money Penalties for the Same Type of Noncompliance
Sections 1819(h)(2)(B)(ii) and 1919(h)(3)(C)(ii) of the Act authorize the Secretary to impose a CMP for each day of noncompliance. Section 1128A(d) of the Act further states that the Secretary shall consider (1) the nature of claims and the circumstances under which they were presented, (2) the degree of culpability, history of prior offenses, and financial condition of the person presenting the claims, and (3) such other matters as justice may require when determining the amount or scope of any penalty. The regulations at § 488.454(d) state that, in the case of a CMP imposed for an instance of noncompliance, the remedy is the specific amount of the CMP imposed for the particular noncompliance deficiency. The meaning of an “instance,” therefore, focuses on a single deficiency citation of the applicable requirements of part 483, subpart B, referenced on the facility's statement of deficiencies (Form CMS-2567) and, under the current regulations, only one type of CMP can be imposed per F tag deficiency.
The statute grants the Secretary broad discretion to determine how appropriate CMPs should be enforced and only limits the imposition to a maximum daily amount. As discussed in the proposed rule, we proposed to expand the circumstances in which a PI CMP can be imposed to allow for more than one PI CMP to be imposed when multiple occurrences, or “instances” of a specific noncompliance are identified during a survey, regardless of whether they are cited at the same regulatory deficiency tag number in the statement of deficiencies.
As previously mentioned, CMS imposes CMPs based on sections 1819(h)(2)(B)(ii) and 1919(h)(3)(C)(ii) of the Act and §§ 488.404 and 488.438 which provides the amount of penalty, the ranges, the basis for penalty amount, increase/decrease of penalty amounts, and factors affecting the amount. While we may impose various enforcement remedies, CMPs are frequently imposed for deficiencies that result in serious injury, harm, impairment, or death to nursing home residents. Currently, we can only impose PI CMPs for different types of noncompliance identified on a survey, while other instances of the same noncompliance would not receive a CMP due to current regulatory limitations.
To strengthen our enforcement policies, we proposed to revise § 488.401 to define “instance” or “instance of noncompliance” as a separate factual and temporal occurrence when a facility fails to meet a participation requirement. We further proposed that each instance of noncompliance would be sufficient to constitute a deficiency and that a deficiency may be comprised of multiple instances of noncompliance. We received combined comments in response to sections IX.B.1 and IX.B.2. A summary of the comments and our responses are listed at the conclusion of section IX.B.2 in this final rule. We received several comments in support of the proposed revision to § 488.401.
2. Imposing Per Instance and Per Day Civil Money Penalties on the Same Survey
As we noted earlier, the Act does not limit the imposition of both a PD and a PI on the same survey, but only limits the total amount a penalty may be imposed for any individual day. Section 488.408(d)(2)(iii) through (iv) and (e)(1)(iii) through (iv) outline the type of remedies that may be imposed based on the severity of the noncompliance. However, these regulations do not state the manner in which the remedies may be imposed.
Because CMPs are designed to spur permanent resolution of deficiencies to maintain resident safety, we believe CMS and the States need flexibility to determine the range of CMPs that can be imposed on facilities that fail to meet the conditions of participation.
As discussed in the proposed rule, we proposed to revise §§ 488.408(e)(2)(ii) and 488.430(a) to expand our authority to impose both a PI CMP and a PD CMP, not to exceed the statutory and regulatory maximum amount on any given day even when combined, when surveyors identify noncompliance. Specifically, in § 488.408(e)(2)(ii), we proposed that for each instance of noncompliance, CMS and the State may impose a PD CMP of $3,050 to $10,000 (as adjusted under 45 CFR part 102), a PI CMP of $1,000 to $10,000 (as adjusted under 45 CFR part 102), or both, in addition to the remedies specified in § 488.408(e)(2)(i). Additionally, we proposed that when a survey contains multiple instances of noncompliance, CMS and the State may impose any combination of per instance or per day CMP for each instance of noncompliance within the same survey. Additionally, we proposed to revise § 488.430(a) to allow for each instance of noncompliance, a PD CMP, PI CMP, “or both” may be imposed, regardless of whether the deficiencies constitute immediate jeopardy. We also proposed to add that when a survey contains multiple instances of noncompliance, a combination of PI and PD CMPs for each instance of noncompliance may be imposed within the same survey.
Additionally, we proposed to make conforming changes by revising § 488.434(a)(2)(iii) to clarify that both PD and PI CMPs can be imposed on the same survey and thus are included in the penalty notice to the facility. Furthermore, we proposed to revise § 488.434(a)(2)(v) to indicate that the date and instance of noncompliance is not a singular event but rather can be multiple “date(s) of the instance(s) of noncompliance.” Lastly, we proposed to revise § 488.440(a)(2) to remove the phrase, “for that particular deficiency,” and replace with, “per instance,” which will allow for more than one PI CMP to be imposed on the same type of noncompliance or “F tag” citation. We sought public comment on these proposed revisions and received over a 100 public comments on these proposals from various parties interested in addressing LTC facilities' issues, including advocacy groups, long-term care ombudsmen, providers and provider industry associations, nursing home staff and administrators, and others. The following is a summary of the comments we received and our responses.
Comment: Several commenters supported the revised definition of “instance(s) of noncompliance” at § 488.401 and the proposed language at § 488.434(a)(2)(v) that indicates instances of the same noncompliance (F-tag) can occur on multiple dates. Commenters also agreed with the revisions at § 488.434(a)(2)(iii), clarifying that both PD and PI CMPs can be imposed simultaneously in the same survey, stating that both CMP types may be warranted based on the facility's noncompliance. Commenters stated that these regulatory changes as proposed, would allow for flexibility in imposing enforcement and align with the goal of enforcement remedies to ensure facility compliance with the Federal participation requirements.
Response: We appreciate the feedback from commenters and agree that by improving the definition of instance(s), our authority to impose multiple PI CMPs and both PI and PD in the same survey will strengthen our enforcement and promote resident safety and quality of care and life.
Comment: Many commenters opposed the change to impose multiple PI CMPs for the same type of noncompliance and PD and PI CMPs in the same survey. One commenter noted that when the scope of a deficiency is cited, it already reflects the extent of the noncompliance when scope and severity are assigned to a deficiency, as doing so may unfairly punish the facility. For example, a PI CMP is imposed based on the scope (isolated, pattern, or widespread) of the cited deficiency, and the revised provision will also allow for multiple PI CMPs imposed at the same scope and severity for each instance of noncompliance. Essentially this commenter noted that the revised process implies that the facility would be fined twice with PI CMPs at the higher scope level of pattern or widespread. Another commenter stated these changes would deviate practices of CMP imposition significantly for nursing homes as compared to other providers, such as hospitals, home health agencies, and hospices causing inconsistencies across enforcement settings. Additionally, they added that the use of CMPs in nursing homes would thus be more extreme than in these other settings.
Response: We disagree with these comments. While the scope and severity level of a deficiency does reflect the extent of the noncompliance, under current regulations, the resultant CMP may not. For example, imposing a single PI CMP may only reflect the scope of a single instance of noncompliance that occurred on a day, but that may not accurately reflect the type of noncompliance and harm to residents that may have occurred on other days. Therefore, the proposed revision will allow CMS to impose CMPs for multiple instances of noncompliance to more accurately reflect the type of noncompliance that occurred on multiple days, and does not represent that a facility would be fined twice at the higher scope and severity level.
Furthermore, in response to comments opposing the imposition of PD and PI CMPs in the same survey, we note that under a PD CMP, a facility may already be fined for each day until the facility is in substantial compliance. This may include the days where specific instances of noncompliance occurred until the facility is determined to be in substantial compliance. The proposed revision gives CMS the ability to also impose a CMP for each instance that noncompliance occurred on different days within that timeframe, rather than a broader CMP that applies to all days from the start of the noncompliance until the facility is in substantial compliance.
These changes are not intended to punish a facility, but rather to ensure the imposition of CMPs, like all enforcement remedies imposed on nursing homes voluntarily choosing to participate in the program, “ensure[s] prompt compliance with program requirements” and are “applied on the basis of noncompliance found during surveys conducted by CMS or by the State survey agency.” 42 CFR 488.402(a) and (b). Congress enacted sections 1819 and 1919 of the Act to provide the Secretary with expansive authority to craft remedies to address noncompliance with Federal standards for nursing home quality care, which is what these revisions are designed to do. The legislative history of the Nursing Home Reform Act of 1987 (NHRA) does not support an assertion that changes cannot be made to the implementing regulations after careful consideration and evaluation of new information, nor that changes cannot be made to encourage achieving and maintaining compliance. Congress has expressly instructed the Secretary that the purpose of “Federal Remedies” is to “assure compliance in Medicaid facilities” with the rules. H.R. Rep. No. 100-391, pt. 1 at 475 (1987). Congress also instructed the Secretary to create penalties that would prevent “yo-yo” or “roller coaster” providers that “correct their deficiencies, and then quickly lapse into noncompliance.” Id. at 471. See also id. at 474 (“The Committee is particularly concerned with the patterns of repeated noncompliance noted by both the [Institute of Medicine] Committee and the GAO.”). As part of this authority, we have found that changes to the implementing regulations are needed to better effectuate the Medicare and Medicaid statutes and overall regulatory enforcement scheme, that is, ensuring providers take all reasonable steps to care for a vulnerable population and help them to “attain or maintain [their] highest practicable physical, mental, and psychosocial well-being.” Sections 1819(b)(2) and 1919(b)(2) of the Act. We are making these revisions precisely because currently repeat noncompliance has been an issue, and these changes will, we hope, remedy that problem.
Because CMPs are designed to spur permanent resolution of deficiencies so that facilities achieve and maintain compliance, we believe CMS and the States need flexibility to determine the range of CMPs that can be imposed on facilities that fail to meet the conditions of participation. For example, if a survey identifies isolated noncompliance that occurred prior to the start of the survey and also identifies separate noncompliance that began and continued to occur during the survey, we are currently unable to impose both a PI CMP and a PD CMP, that are within the requisite daily limits to address these two separate occurrences of noncompliance identified during the same survey. In other words, if a survey identified numerous instances of medication administration errors as well as systemic noncompliance with infection control policies, we believe imposing a PI CMP for the medication errors and a PD CMP for the infection control deficiencies, in this general example, could be a more effective enforcement response to both the isolated medication noncompliance incidents from prior to the survey and the current noncompliance with infection control policies. Due to the additional instances of noncompliance identified, a PD CMP that covers the noncompliance with infection control requirements alone may not encourage the facility to sustain compliance with medication administration. Without this type of flexibility, CMS cannot impose remedies that are sufficient to ensure that any systemic issues that caused the noncompliance are permanently corrected. Moreover, we have found that the failure of nursing homes to take the necessary steps to permanently resolve systemic problems increases the probability that deficiencies will recur, progressing to a higher scope and severity that ultimately results in harm or increased harm to residents. For example, if noncompliance occurred on a date prior to the start of a survey, and noncompliance was also identified during the survey, under the current structure, CMS could impose a PD CMP that would start accruing from the first date of noncompliance. Under the new revision, CMS could impose a PI CMP for the noncompliance that occurred prior to the survey, and PD CMP for the noncompliance that was identified during the survey. This will allow CMS to impose a CMP that is commensurate with the actual noncompliance that occurred, rather than having the CMP amount be impacted by the timing of the survey.
We also disagree that there is an issue in the application of CMPs for nursing homes as compared to other providers. CMPs for noncompliance with program participation requirements are not an available remedy for hospitals. Though they are available for home health agencies and hospices, unlike these providers, the NHRA is a nursing home specific statute in which Congress has expressly instructed the Secretary to pay especial attention to nursing home compliance with the standards of participation in order to ensure that facilities not simply meet the conditions of participation, but also comply with the statutory mandate that nursing homes must provide services and activities to “attain or maintain the highest practicable physical, mental, and psychosocial well-being of each resident” and in such manner and such environment that will “promote maintenance or enhancement of the quality of life of each resident.” Sections 1819(b)(1), 1819(b)(2), 1919(b)(1), and 1919(b)(2) of the Act (emphasis added). Other providers have very different conditions for participation and enforcement of those conditions. The revisions in this rule are to ensure that nursing homes comply to the unique requirements for participation for long term care facilities.
Comment: Commenters questioned the necessity of the revisions to impose PD and PI CMPs in the same survey and multiple PI CMPs for the same type of noncompliance. They note that CMS has existing enforcement authority to impose a per day CMP amount up to the regulatory maximum as adjusted by the 2015 Act. As such, the commenter expressed concerns that CMS could use the regulatory revisions to impose multiple CMPs that exceed the daily regulatory maximum.
Response: We thank the commenter for their comment. As noted in the proposed rule and the preamble of this final rule, CMS recognizes that the statute limits the daily amount of a CMP imposition up to the regulatory maximum in accordance with § 488.408, as adjusted by the 2015 Act. Additionally, given that the timing of a revisit survey can vary and potentially result in a disparate CMP total among facilities for similar noncompliance, even when the noncompliance may have resulted in relatively less harm to residents, we believe these revisions would allow for improved consistency in the imposition of CMPs. Also, the regulatory revisions will provide CMS additional flexibility to impose CMPs at an amount that aligns with the severity of the noncompliance, but that does not exceed the statutory and regulatory maximum amount on a given day.
Comment: Many commenters objected to the CMP proposals which they described as an expansion, which the commenters believed may divert a facility's funds away from recruiting and retaining direct care staff to meet the new minimum nursing home staffing requirements that would help improve resident quality of care. Commenters referenced the statements on Improving Safety and Quality in the Nation's nursing homes,[107] which outlined a set of reforms including assuring that every nursing home provides a sufficient number of staff who are adequately trained to provide high-quality care. There is concern with how these CMP enforcement updates will interact with the finalized minimum staffing requirements for long-term care facilities. One commenter also expressed an additional concern that increased financial penalties may lead to additional facility closures and create issues related to access to care.
Response: We thank the commenters for their comments. The “Minimum Staffing Standards for Long-Term Care (LTC) Facilities and Medicaid Institutional Payment Transparency” final rule [108] was issued on April 22, 2024. This final rule establishes minimum nurse staffing requirements, which aim to significantly reduce the risk of residents receiving unsafe and low-quality care within LTC facilities. The enforcement of the new staffing requirements will not begin until those requirements are implemented, which is staggered over time; the relevant implementation dates are provided in the final rule. The revisions to the enforcement regulations in this final rule, however, will adjust our ability to impose PD and PI CMPs for noncompliance with any requirement and are not exclusive to the new staffing requirements. CMS has a statutory obligation to assure the enforcement of Federal requirements are adequate to protect the health, safety, welfare and rights of residents. Enforcement remedies, such as CMPs, address noncompliance with any requirement, and these revisions intend to improve our ability to do so in a more targeted and effective manner. We further note that the revisions to the CMP authorities are not intended to cause an increase of facility closures or create any access to care issues. As per § 488.438(f)(2), when choosing to impose a CMP remedy, CMS considers a facility's financial condition, among other factors. CMS remains focused on improving the health and safety of nursing home residents by ensuring quality care and ensuring access to care. Reforming the CMP system can further help to improve the quality and safety of care that residents in SNFs and NFs receive by incentivizing facility violations to be remedied faster.
Comment: CMS received a comment stating concerns that CMS will be assessing more CMPs while suggesting CMS include a limit of $5,000 on projects submitted to the Civil Money Penalty Reinvestment Program (CMPRP). The commenter notes that “although we understand the importance of CMPs as an enforcement tool, we believe that the combination of these changes will remove even more funding from the nursing home sector at the same that CMS has made it extremely challenging to use those funds for their intended purpose of protecting or improving resident care.”
Response: This comment regarding the CMPRP project limits is outside the scope of this final rule; however, we note that the proposed revisions to §§ 488.430(a) and 488.434(a)(2)(iii) do not impact facilities' ability to apply for or receive grants through the CMPRP for eligible quality improvement programs that benefit residents.
Comment: Commenters also articulated concerns regarding consistency in the survey process, stating, “survey findings can vary significantly regardless of the actual instances of noncompliance.”
Response: We appreciate the commenters' concerns. However, all surveyors are required to use CMS published protocols and interpretive guidance for the regulatory requirements when assessing a facility's compliance with Federal requirements. Noncompliance citations are based on violations of the regulations, which are based on observations of the nursing home's performance or practices as well as record review and interviews. We acknowledge that there are occasional variations in survey findings due to the unique facts and circumstances of each individual situation. However, while CMPs are imposed based on survey findings, we believe this rule may actually improve CMS' ability to impose CMPs in a more consistent manner nationwide and in a manner that better aligns with the severity of the noncompliance that occurred.
After consideration of public comments, we are finalizing the revisions as proposed. This final rule is effective 60 days after it is published in the Federal Register . These requirements will be operationalized beginning March 3, 2025. This will allow CMS to make the corresponding changes in our systems (iQIES) while we are transitioning to a new technology platform, and to provide the necessary training to implement these changes.
3. Timing of Enforcement
Sections 1819(h)(2)(A) and 1919(h)(1) and (3) of the Act state that when a facility is found to be in compliance with the requirements but “. . . as of a previous period, did not meet such requirements,” the Secretary and the State may impose a CMP for the days that the facility is found out of compliance with the requirements. The regulation at § 488.430(b) states that “CMS or the State may impose a civil money penalty for the number of days of past noncompliance since the last standard survey, including the number of days of immediate jeopardy.”
As discussed in the proposed rule, due to an increase in the number of complaint surveys being conducted (for example, over 10,000 additional surveys since 2015) and resulting increased enforcement actions, the current regulation may result in an unanticipated limit on CMS's authority to impose remedies for the noncompliance deficiencies identified when the last standard survey was performed. For example, a complaint survey might need to be conducted shortly after a standard survey, not leaving enough time to impose a CMP for deficiencies identified in the first survey before the second survey is concluded because the regulation limits how far back CMS or the State may go when calculating a CMP amount: since the last standard survey. We proposed to revise § 488.430(b) by changing “since the last standard survey” to “since the last three standard surveys.” We believe this proposed revision aligns with the statutory mandate that the Secretary ensure that enforcement remedies ensure quality care and adequately protect the health and safety of nursing home residents in facilities where the Medicare and/or Medicaid programs pay for services. These proposed revisions are designed to enable CMS or State survey agencies to impose a variety of CMPs for noncompliance, particularly when surveyors have identified deficiencies during one survey that cannot be addressed because, for example, a subsequent survey has taken place. In these situations, it is important for CMS and the State to be able to impose a CMP (per day, per instance, or both), as warranted, to help ensure that the facility's correction is swift and its compliance is permanent. Additionally, as discussed in the proposed rule, limiting the imposition of CMPs for noncompliance that occurred and was cited since the last three standard surveys is more reflective of a facility's current compliance performance and is consistent with current CMS practices of posting survey results from the last three standard surveys and last three years of complaint surveys on Nursing Home Care Compare as well as the Nursing Home Five Star Quality Rating System.
We sought public comments on this proposal and an alternative look-back period that would also ensure CMPs are imposed in a manner that is not dependent on when the next standard survey is conducted. There were no comments regarding an alternative look-back period. The following is a summary of the comments we received and our responses.
Comment: Some commenters supported the revision to § 488.430(b) that authorizes the imposition of CMPs for noncompliance that was previously cited since the last three standard surveys.
Response: We appreciate the support for this proposal and thank the commenters for their comments.
Comment: We also received comments questioning how this revision would be used to enforce new regulations such as the “Minimum Staffing Standards for Long-Term Care (LTC) Facilities and Medicaid Institutional Payment Transparency” final rule.[109]
Response: As stated previously, the enforcement of the new requirements for minimum staffing standards will not begin until the requirements become effective; the relevant effective and implementation dates are stated in the final rule. The revisions in this final rule will enable CMS to look-back three standard surveys for any noncompliance that was previously cited but no CMP was yet imposed and will allow for imposition of CMPs. The revision's intent is not to instruct that surveyors look-back to the last three standard surveys for noncompliance that was not previously cited. The revisions will not impact the new staffing regulations any differently than they impact CMS' ability to impose CMPs for any other noncompliance where the imposition of a CMP is warranted.
Comment: We received comments voicing concerns about how the proposed revisions would be affected by the current survey backlog. The commenters are concerned that facilities affected by the survey backlog should not be penalized with a lengthy lookback period when they have no ability to change it. Additionally, in the current environment where some States are using contracted surveyors and there is inconsistency, the commenter believes it is inequitable to apply a national standard that could penalize some States.
Response: We thank the commenters for their concerns, but we disagree. We wish to clarify that the proposal to look-back to the last three standard surveys pertains only to CMPs issued as part of CMS' oversight and enforcement of regulatory noncompliance that occurred and was specifically cited in a previous period, but no CMP was yet imposed. This regulatory revision is not intended to create a new ability for surveyors to investigate and cite potential or alleged noncompliance that occurred during the proposed look-back period that had not already been cited and included on a Statement of Deficiencies. The intent of the proposed revision is to ensure the imposition of CMPs, when warranted as an enforcement response, is equitable and that all providers, regardless of their location will be subject to the same amount of enforcement in accordance with the CMP Analytic Tool.[110] This revision allows CMS to impose a variety of CMPs, as necessary, for regulatory noncompliance that occurred in a previous period even if a subsequent survey has taken place. We do note however, that the current regulatory scheme still requires that CMS investigate any received complaints, without any temporal limitation on the specific alleged deficiencies complained of, and thus the possibility of investigations into allegations during the proposed look-back period is possible. See42 CFR 488.308(f).
After consideration of public comments we received and for the reasons discussed earlier in this section and in the proposed rule, we are finalizing the proposed revision with two modifications at § 488.430(b). First, we are replacing “past noncompliance” with “previously cited noncompliance” as we are concerned that stakeholders are confusing the reference to past noncompliance with noncompliance that occurred and was already previously cited on a Statement of Deficiencies that was issued to a provider. Therefore, as discussed earlier in this section, “previously cited noncompliance” means noncompliance that was already previously cited on a Statement of Deficiencies that was issued to a provider for a survey that occurred since the last three standard surveys but a CMP has not yet been imposed. Also, as previously stated, this regulatory revision is not intended to create a new ability for surveyors to investigate and cite potential or alleged noncompliance that occurred during the proposed look-back period that had not already been cited and included on a Statement of Deficiencies.
Second, we proposed that CMS or the State may impose a civil money penalty for the “number of days” of previously cited noncompliance, but are adding, “or instances,” as a conforming change to specify that either a PD or PI CMP, or both, may be imposed for previously cited noncompliance, consistent with the revisions that are finalized in this rule. This final rule is effective 60 days after it is published in the Federal Register . These requirements will be operationalized beginning March 3, 2025. This will allow CMS to make the corresponding changes in our system while we are transitioning to a new technology platform (iQIES), and to provide the necessary training to implement these changes.
X. Collection of Information Requirements
Under the Paperwork Reduction Act of 1995 (PRA), we are required to provide 30-day notice in the Federal Register and solicit public comment before a collection of information requirement is submitted to the Office of Management and Budget (OMB) for review and approval. In order to fairly evaluate whether an information collection should be approved by OMB, section 3506(c)(2)(A) of the Paperwork Reduction Act of 1995 requires that we solicit comment on the following issues:
- The need for the information collection and its usefulness in carrying out the proper functions of our agency.
- The accuracy of our estimate of the information collection burden.
- The quality, utility, and clarity of the information to be collected.
- Recommendations to minimize the information collection burden on the affected public, including automated collection techniques.
We solicited public comment on each of these issues for the following sections of this document that contain information collection requirements (ICRs):
Using the following format describe the information collection requirements that are in each section.
A. Information Collection Requirements (ICRs)
1. ICRs Regarding the Skilled Nursing Facility Value-Based Purchasing Program
We are not removing or adding any new or revised SNF VBP measure-related requirements or burden in this rule. Consequently, this final rule does not set out any new SNF VBP-related collections of information that would be subject to OMB approval under the authority of the PRA.
2. ICRs Regarding the Skilled Nursing Facility Quality Reporting Program (SNF QRP)
In accordance with section 1888(e)(6)(A)(i) of the Act, the Secretary must reduce by 2-percentage points the otherwise applicable annual payment update to a SNF for a fiscal year if the SNF does not comply with the requirements of the SNF QRP for that fiscal year.
As stated in section VI.C.3. of the proposed rule and VII.C.3. of this final rule, we proposed to adopt four new items as standardized patient assessment data elements under the SDOH category and modify one item collected as a standardized patient assessment data element under the SDOH category beginning with the FY 2027 SNF QRP. In section VI.E.3. of the proposed rule and VII.E.3. of this final rule, we also proposed that SNFs participating in the SNF QRP, be required to participate in a validation process. Specifically, we proposed adopting a similar validation process for the SNF QRP that we adopted for the SNF VBP beginning with the FY 2027 SNF QRP.
As stated in section VI.C.3. of the proposed rule and section VII.C. of this final rule, we proposed to adopt four new items as standardized patient assessment data elements under the SDOH category and modify one item collected as a standardized patient assessment data element under the SDOH category beginning with the FY 2027 SNF QRP. The proposed new and modified items would be collected using the MDS. The MDS, in its current form, has been approved under OMB control number 0938-1140. Four items would need to be added to the MDS at admission to allow for collection of these data, and one would be modified. Additionally, as stated in section VI.E.2. of the proposed rule and section VII.E.2. of this final rule, we are finalizing our proposal to require SNFs to collect and submit data on the four new and one modified SDOH standardized patient assessment data elements at admission beginning October 1, 2025. However, we are finalizing a modification to the data specifications of the new and modified SDOH items so that they exclude any SNF residents who, immediately prior to their hospitalization that preceded a new SNF stay, resided in a NF for at least 366 continuous days. SNFs can monitor the MDS 3.0 Technical Information web page at https://www.cms.gov/medicare/quality/nursing-home-improvement/minimum-data-set-technical-information for updates.
The net result of collecting four new items at admission and modifying the Transportation item (including the modification that this item be collected at admission only, rather than at admission and discharge) is an increase of 0.9 minutes or 0.015 hour of clinical staff time at admission [(4 items × 0.005 hour) minus (1 item × 0.005 hour)]. We identified the staff type based on past SNF burden calculations, and our assumptions are based on the categories generally necessary to perform an assessment. We believe the new and modified items will be completed equally by a Registered Nurse (RN) and Licensed Practical and Licensed Vocational Nurse (LPN/LVN). However, individual SNFs determine the staffing resources necessary.
For the purposes of calculating the costs associated with the collection of information requirements, we obtained median hourly wages for these staff from the U.S. Bureau of Labor Statistics' (BLS) May 2022 National Occupational Employment and Wage Estimates.[111] To account for other indirect costs and fringe benefits, we doubled the hourly wage. These amounts are detailed in Table 34. We established a composite cost estimate using our adjusted wage estimates. The composite estimate of $65.31/hr was calculated by weighting each hourly wage equally [($78.10/hr × 0.5) plus ($52.52/hr × 0.5) = $65.31].
We estimate that the burden and cost for SNFs for complying with requirements of the FY 2027 SNF QRP will increase under this requirement to collect and submit these new and modified items on the MDS for each resident at admission. Therefore, we are providing a revised estimate of burden and cost from what we estimated in section IX.A.2. of the proposed rule. Using FY 2023 data, we estimate 199,856 5-day PPS assessments would be impacted by the modification within the MDS data specifications in order to decrease the burden of capturing this information on any SNF residents who, immediately prior to their hospitalization that preceded a new SNF stay, resided in a NF for at least 366 continuous days. As a result, we estimate a new total of 1,766,806 admissions. Our estimate of planned discharge assessments is not changing and remains at 754,287 planned discharges. We are changing the number of SNFs based on more recent information and more recent provider to CBSA matching from 15,393 SNFs annually to 15,477 SNFs annually. The result is a revised increase of 30,565.41 hours in burden for all SNFs [(1,766,806 5-day PPS assessments × 0.02 hour for the four new SDOH items) minus [(199,856 5-day PPS assessments × 0.005 hour for the modified Transportation item) plus (754,287 planned discharges × 0.005 hour)]], reflecting a reduction of 4,996.41 hours from the estimate in the proposed rule (89 FR 23424). Given 0.02 hour at $65.31 per hour to complete an average of 114 5-day PPS assessments per provider per year minus the sum of 0.005 hour at $65.31 per hour to complete an average of 12.91 5-day PPS assessments per provider per year and 0.005 at $65.31 per hour to complete an average of 49 Planned Discharge assessments, we estimate the total cost would be increased by $128.98 per SNF annually, or $1,996,226.60 for all SNFs annually, a reduction of $21.90 per SNF annually or $326,314.88 for all SNFs annually from the estimate in the proposed rule (89 FR 23424). The increase in burden will be accounted for in a revised information collection request under OMB control number (0938-1140). The required 60-day and 30-day notices would publish in the Federal Register and the comment periods will be separate from those associated with this rulemaking.
In summary, under OMB control number (0938-1140), as a result of finalizing the policies in this final rule, we estimate the SNF QRP will result in an overall increase of 30,565.41 hours annually for 15,477 SNFs. The total revised cost increase related to this information collection is approximately $1,996,226.60 and is summarized in Table 35.
We invited public comments on the proposed information collection requirements. We have summarized the comments we received in section VII.E.2 of this final rule and provided responses. After careful consideration of the public comments we received, we are finalizing our proposal with modification as stated above.
3. ICRs Regarding the Minimum Data Set (MDS) Beginning October 1, 2025
The MDS is used for meeting the SNF Requirements of Participation, requirements under the SNF QRP, and for payment purposes under the SNF PPS. As outlined in the FY 2019 SNF PPS final rule (83 FR 39165 through 39265), several MDS items are not needed in case-mix adjusting the per diem payment for PDPM. However, they were not accounted for in the FY 2019 SNF PPS final rule. Therefore, we are removing these items from the 5-day Medicare-required assessment beginning October 1, 2025. We have provided an estimate of the reduction in burden here and in Table 36. The items to be removed are:
- O0400.A.1. Speech-Language Pathology and Audiology Services; Individual minutes.
- O0400.A.2. Speech-Language Pathology and Audiology Services; Concurrent minutes.
- O0400.A.3. Speech-Language Pathology and Audiology Services; Group minutes.
- O0400.A.3A. Speech-Language Pathology and Audiology Services; Co-treatment minutes.
- O0400.A.4. Speech-Language Pathology and Audiology Services; Days.
- O0400.A.5. Speech-Language Pathology and Audiology Services; Therapy start date.
- O0400.A.6. Speech-Language Pathology and Audiology Services; Therapy end date.
- O0400.B.1. Occupational Therapy; Individual minutes.
- O0400.B.2. Occupational Therapy; Concurrent minutes.
- O0400.B.3. Occupational Therapy; Group minutes.
- O0400.B.3A. Occupational Therapy; Co-treatment minutes.
- O0400.B.4. Occupational Therapy; Days.
- O0400.B.5. Occupational Therapy; Therapy start date.
- O0400.B.6. Occupational Therapy; Therapy end date.
- O0400.C.1. Physical Therapy; Individual minutes.
- O0400.C.2. Physical Therapy; Concurrent minutes.
- O0400.C.3. Physical Therapy; Group minutes.
- O0400.C.3A. Physical Therapy; Co-treatment minutes.
- O0400.C.4. Physical Therapy; Days.
- O0400.C.5. Physical Therapy; Therapy start date.
- O0400.C.6. Physical Therapy; Therapy end date.
- O0400.E.2. Psychological Therapy; Days.
The net result of removing the collection of these items is a decrease of 6.6 minutes of clinical staff time at admission. We believe that these items are completed equally by a RN and LPN/LVN. Individual SNFs determine the staffing resources necessary.
For the purposes of calculating the costs associated with the collection of information requirements, we obtained median hourly wages for these staff from the BLS May 2022 National Occupational Employment and Wage Estimates.[112] To account for other indirect costs and fringe benefits, we have doubled the hourly wage. These amounts are detailed in Table 36. We established a composite cost estimate using our adjusted wage estimates. The composite estimate of $65.31/hr was calculated by weighting each hourly wage equally [($78.10/hr × 0.5) plus ($52.52/hr × 0.5) = $65.31].
Using FY 2023 data, we estimate a total of 1,966,662 admissions to 15,477 SNFs annually. This equates to a decrease of 216,332.82 hours in burden for all SNFs. Given 0.11 hour at $65.31 per hour to complete an average of 127 5-day PPS assessments per provider per year, we estimate the total cost will be decreased by $912.88 per SNF annually, or $14,128,696.47 for all SNFs annually.
As noted previously in this section of the final rule, we did not formally propose the changes to the MDS. Rather we used this opportunity to provide SNFs the information collection requirements associated with a change that was not accounted for in the FY 2019 SNF PPS final rule. We received a limited number of comments about this notification, and are providing a summary of those here, with our responses.
Comment: Three commenters supported the removal of several MDS items that are not needed in case-mix adjusting the per diem payment for PDPM but were not accounted for in the 2019 SNF PPS. These commenters acknowledged CMS' efforts to reduce provider burden. One of these commenters appreciated that CMS was not removing the Therapy items in Section O on the PPS Discharge Assessment that collect the number of physical, occupational, and speech-language pathology and audiology minutes provided since the start date of the resident's most recent Medicare Part A stay.
Response: We appreciate the support from commenters and agree that removing the requirement to collect the data at the time of the Medicare Part A admission, while retaining the requirement to collect the data at the time of discharge from the Medicare Part A stay, balances the need to monitor the data, while also minimizing provider burden.
Comment: Several commenters urged CMS not to remove these items from the 5-day PPS assessment because it gave the appearance that rehabilitation therapy was being devalued and CMS would not be able to track functional outcomes. Two of these commenters suggested that there are not enough safeguards in place to ensure patients receive the appropriate skilled therapy they need to achieve desired outcomes, and one of these commenters suggested the therapy minutes items provided a trigger for nursing staff to consider whether therapy should be implemented. One of the commenters stated it is too early to eliminate the items from the MDS given that PDPM was implemented approximately 5 years ago. Other commenters noted that they were concerned that without these minutes documented, residents may only receive “low” skilled therapies. Finally, one of the commenters stated collection of these items allows CMS to ensure that when they make a therapy payment, therapy services are delivered.
Response: We acknowledge the commenters concerns, and it is not our intent to devalue therapy. In fact, functional outcomes are a key component of our SNF QRP measure set, including the Discharge Function Score measure that was adopted in the FY 2024 SNF PPS final rule (88 FR 53233 through 53243). As we stated at the time, the implementation of interventions that improve residents' functional outcomes and reduce the risks of associated undesirable outcomes as a part of a resident-centered care plan is essential to maximizing functional improvement. For many people, the overall goals of SNF care may include optimizing functional improvement, returning to a previous level of independence, maintaining functional abilities, or avoiding institutionalization (88 FR 53234). We take the quality of care residents receive in SNFs seriously, and monitor the impact of policy decisions, including adding or removing quality measures and assessment items. We do not believe it is necessary to retain these items on the 5-day PPS admission assessment to trigger a decision as to whether therapy services are needed. SNFs have a responsibility to develop and implement a baseline care plan for each resident that includes the instructions needed to provide effective and person-centered care of the resident that meet professional standards of quality care (§ 483.21(a)). Additionally, the facility must develop and implement a comprehensive person-centered care plan for each resident (§ 483.21(b)) that has been prepared by an interdisciplinary team (§ 483.21(b)(2)(ii)). The comprehensive person-centered care plan must include the services to be furnished in order to attain or maintain the resident's highest practicable physical, mental, and psychosocial well-being as required under § 483.24, § 483.25, or § 483.40.
We believe retaining the therapy items on the PPS discharge assessment will achieve the same goals, but with less burden on SNFs. Specifically, we will still collect the total number of individual, concurrent, group, and cotreatment therapy minutes by discipline, as well as the number of days of each therapy discipline a resident received over the course of their Part A stay. Therefore, we will be able to ensure there is no significant change in the intensity of therapy a resident receives and understand the relationship between the delivery of therapy services with functional outcomes.
Regarding the comment that residents may receive “low” skilled therapies, we are unclear how to interpret what the commenter may have been referring to as “low” skilled therapies. Medicare only has one definition of skilled therapy,[113] and the MDS RAI manual has consistently provided guidance to SNFs that the number of days and minutes recorded on the MDS may only include the skilled therapy treatment time. And, as noted previously in this final rule, SNFs have a responsibility to provide the necessary care and services to attain or maintain the highest practicable physical, mental, and psychosocial well-being, in accordance with the comprehensive assessment and plan of care (42 CFR 483.25). Regarding the comment that CMS will be unable to ensure that when they make a therapy payment, therapy services are delivered, we remind commenters that the SNF PPS does not use the number of therapy minutes to determine SNF payment. The SNF PDPM was implemented on October 1, 2019, replacing the Resource Utilization Groups (RUG) which was dependent on Section O for therapy minutes. The PDPM consists of five case-mix adjust components, all based on data-driven, interested parties-vetted patient characteristics, rather than therapy utilization minutes.
Comment: Two commenters urged CMS to continue tracking the therapy start date, which is only collected on the 5-day PPS assessment, since this datapoint may be useful for research on best practices and functional outcomes, including determining whether or how delays in the start of rehabilitation care may impact patient outcomes and discharge disposition.
Response: We thank these commenters for their input. However, CMS no longer uses start dates because the data are not needed for Federal governmental purposes. As we noted in the FY 2019 SNF PPS final rule, we closely monitor service utilization, payment, and quality trends when evaluating patient care outcomes.
Comment: One commenter stated the therapy start date is necessary to retain since it is used in calculating the Discharge Function Score measure, and requested CMS clarify how this measure would be calculated without the data point.
Response: The Discharge Function Score measure does not use the O0400A5 Speech-Language Pathology and Audiology Services Start date, the O0400B5 Occupational Therapy Services Start date, or the O0400C5 Physical Therapy Services Start date in the calculation. Therefore, these data will have no effect on the calculation of the measure scores.
Comment: One commenter recognized that removing items from the MDS reduces administrative burden but noted that CMS overestimated the amount of time that it takes to track therapy utilization using the MDS tool and did not agree that the collection and submission of these items takes more than 6 minutes of staff time per patient at admission.
Response: The commenter did not provide specific information to support why they believe the burden was overestimated. The 6.6 minutes per MDS is based on past MDS burden calculations and represents the time it takes to encode the MDS. Our assumptions for staff type were based on the categories generally necessary to perform an assessment, and subsequently encode it, and is consistent with past collection of information estimates.
After careful consideration of the public comments we received, we are finalizing our intention to remove the Section O0400 items identified above from the MDS.
4. ICRs Regarding the Proposal for SNFs To Participate in a Validation Process
In section VI.E.3. of the proposed rule, we proposed to require SNFs to participate in a validation process beginning with the FY 2027 SNF QRP. We provided an estimate of burden in Table 37, and noted that the increase in burden will be accounted for in a new information collection request.
As stated in section VI.E.3(a) of the proposed rule and section VII.E.3(a) of this final rule, we proposed to require SNFs to participate in a validation process for assessment-based measures beginning with the FY 2027 SNF QRP. We identified the staff type based on past SNF burden calculations, and our assumptions are based on the categories generally necessary to perform an assessment. We believe that the medical records will be collected and submitted by a Medical Records and Health Information Technologist and Medical Registrar (HIT/MR). However, individual SNFs determine the staffing resources necessary. For the purposes of calculating the costs associated with the collection of information requirements, we obtained median hourly wages for these staff from the BLS May 2022 National Occupational Employment and Wage Estimates.[114] To account for other indirect costs and fringe benefits, we doubled the hourly wage to establish an adjusted wage estimate of $56.02/hr. These amounts are detailed in Table 37.
We proposed that our validation contractor will select, on an annual basis, up to 1,500 SNFs and up to 10 medical records from each of the selected SNFs. We proposed that the selected SNFs will have the option to submit digital or paper copies of the requested medical records to the validation contractor.
For the purposes of burden estimation, we assume all the activities associated with the SNF QRP validation process will be completed by a HIT/MR. For selected SNFs utilizing electronic health records (EHR), we anticipate an increase of 3 hours up to 7.5 hours of HIT/MR time per SNF to submit a sample of up to 10 records. For selected SNFs that do not utilize EHRs, we anticipate an increase of 5 hours up to 12.5 hours of HIT/MR time per SNF to submit a sample of up to 10 records. Additionally, SNFs that do not utilize EHRs may incur printing and shipping costs if they are unable to submit the records via an electronic portal, and for these SNFs, we estimate the cost to print and ship a sample of up to 10 records would range from $842.67 up to $4,114.35.
We also anticipate that a sample of up to 10 medical records will consist of SNF stays that vary in length of stay. We estimate the length of stay for each of the selected medical records could range from 20 days (or less) up to or exceeding 366 days. For purposes of our burden estimate, we anticipate the average sample of up to 10 medical records will be distributed among the possible lengths of stay (that is, approximately 40 percent of stays or 4 stays would be 1 to 30 days, 40 percent of stays or 4 stays would be 31 to 100 days, and 20 percent of stays or 2 stays would last 101 to 366 or more consecutive days). We also estimate that approximately 85 percent of nursing homes utilize some form of EHRs.[115] Therefore, we estimate the total cost to submit up to 10 medical records will range between $335,699.85 and $477,368.10 for all 1,500 SNFs selected, depending on the length of stay of the sample medical records and whether the SNFs use an EHR. We also estimate that total cost to submit up to 10 medical records will range between $263.29 [$335,699.85/(1,500 × 0.85 SNFs)] and $2,121.64 [$477,368.10/(1,500 × 0.15 SNFs)] per SNF selected depending on the length of stay of the sample of medical records and whether the SNF uses an EHR. On average we estimate the total cost will be increased by $813,067.95 for all 1,500 selected SNFs [[($263.29 × (1,500 × 0.85)] plus [$2,121.64 × (1,500 × 0.15)]] and $542.05 per selected SNF ($813,067.95/1,500 SNFs) annually.
In section VI.E.3(b). of the proposed rule and section VII.E.3.(b) of this final rule, we proposed to require SNFs to participate in a validation process for Medicare fee-for-service claims-based measures beginning with the FY 2027 SNF QRP. All Medicare fee-for-service claims-based measures are already reported to the Medicare program for payment purposes, and therefore there is no additional burden for SNFs.
We invited public comments on the proposed information collection requirements. We have summarized the comments we received in section VII.E.3 of this final rule and provided responses. After careful consideration of the public comments received, and for the reasons outlined in this section of the final rule and our comment responses, we are finalizing the requirements as proposed.
5. ICRs Regarding Nursing Home Enforcement
This rule finalizes our proposals to expand and strengthen enforcement processes to increase CMS' flexibility when imposing CMPs. While Omnibus Budget Reconciliation Act of 1987 (OBRA '87) exempts nursing home enforcement requirements from the PRA, the anticipated increase in penalties due to facility noncompliance being cited are quantified in the regulatory impact analysis (RIA) section of this preamble.
XI. Economic Analyses
A. Regulatory Impact Analysis
1. Statement of Need
a. Statutory Provisions
This rule updates the FY 2025 SNF prospective payment rates as required under section 1888(e)(4)(E) of the Act. It also responds to section 1888(e)(4)(H) of the Act, which requires the Secretary to provide for publication in the Federal Register before the August 1 that precedes the start of each FY, the unadjusted Federal per diem rates, the case-mix classification system, and the factors to be applied in making the area wage adjustment. These are statutory provisions that prescribe a detailed methodology for calculating and disseminating payment rates under the SNF PPS, and we do not have the discretion to adopt an alternative approach on these issues.
With respect to the SNF QRP, we proposed and are finalizing several updates beginning with the FY 2027 SNF QRP as described in section VII. of this final rule. Specifically, we are finalizing our proposal to collect four new items as standardized patient assessment data elements under the SDOH category and modify one item collected as a standardized patient assessment data element under the SDOH category in the MDS beginning with the FY 2027 SNF QRP with one modification. Specifically, we are finalizing the data specifications of the new and modified SDOH items so that they exclude any SNF residents who, immediately prior to their hospitalization that preceded a new SNF stay, resided in a NF for at least 366 continuous days. We believe these new and modified items advance the CMS National Quality Strategy Goals of equity and engagement by encouraging meaningful collaboration between healthcare providers, caregivers, and community-based organizations to address SDOH prior to discharge from the SNF. We also are finalizing our proposal to adopt a validation process for the SNF QRP beginning with the FY 2027 SNF QRP with modification. Specifically, we are finalizing that our validation contractor will select, on an annual basis, up to 1,500 SNFs that submit at least one MDS record in the FY 2 years prior, rather than the CY 3 years prior, to the applicable FY SNF QRP. We believe this validation process satisfies section 111(a)(4) of Division CC of the Consolidated Appropriations Act, 2021 (Pub. L. 116-260) which requires that the data submitted under the SNF QRP (section 1888(e)(6) of the Act) be subject to a validation process. We are also finalizing revisions to our regulation at § 413.360.
With respect to the SNF VBP Program, this final rule updates SNF VBP Program requirements for FY 2025 and subsequent years. Section 1888(h)(3) of the Act requires the Secretary to establish and announce performance standards for SNF VBP Program measures no later than 60 days before the performance period, and this final rule includes numerical values of the performance standards for the FY 2027 program year for the SNFRM, SNF HAI, Total Nurse Staffing, Nursing Staff Turnover, Falls with Major Injury (Long-Stay), DC Function, and Long Stay Hospitalization measures; and numerical values of the performance standards for the FY 2028 program year for the DTC PAC SNF and SNF WS PPR measures. We are also required under section 1888(h)(1)(C) of the Act to establish a minimum number of measures that apply to a facility for the applicable performance period. Therefore, we are finalizing the measure minimum for the FY 2028 program year and subsequent program years, which will be the same as the measure minimum we previously finalized for the FY 2027 program year (88 FR 53303).
b. Discretionary Provisions
In addition, this final rule includes the following discretionary provisions:
(1) SNF Market Basket Adjustment
We are rebasing and revising the SNF market basket to reflect a 2022 base year. Since the inception of the SNF PPS, the market basket used to update SNF PPS payments has been periodically rebased and revised to reflect more recent data. We last rebased and revised the market basket applicable to the SNF PPS in the FY 2022 SNF PPS final rule (86 FR 42444 through 42463) where we adopted a 2018-based SNF market basket.
Given changes to the industry in recent years and public comments about the timeliness of the weights, we have been monitoring the Medicare cost report data to determine if a more frequent rebasing schedule than our standard schedule (which has generally been about every 4 years) is necessary. In light of this analysis, we are incorporating data that is more reflective of recent SNF expenses.
(2) SNF Forecast Error Adjustment
Each year, we evaluate the SNF market basket forecast error for the most recent year for which historical data is available. The forecast error is determined by comparing the projected SNF market basket increase each year with the actual SNF market basket increase in that year. In evaluating the data for FY 2023, we found that the forecast error for that year was 1.7 percentage points, exceeding the 0.5 percentage point threshold we established in regulation to trigger a forecast error adjustment. Given that the forecast error exceeds the 0.5 percentage point threshold for FY 2023, current regulations require that the SNF market basket percentage increase for FY 2025 be adjusted upward by 1.7 percentage points to account for forecasting error in the FY 2023 SNF market basket update.
(3) Technical Updates to ICD-10 Mappings
In the FY 2019 SNF PPS final rule (83 FR 39162), we finalized the implementation of the PDPM, effective October 1, 2019. The PDPM utilizes ICD-10 codes in several ways, including using the patient's primary diagnosis to assign patients to clinical categories under several PDPM components, specifically the PT, OT, SLP, and NTA components. In this rule, we are finalizing several substantive changes to the PDPM ICD-10 code mapping.
2. Introduction
We have examined the impacts of this final rule as required by Executive Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563 on Improving Regulation and Regulatory Review (January 18, 2011), Executive Order 14094, entitled “Modernizing Regulatory Review” (April 6, 2023), the Regulatory Flexibility Act (RFA, September 19, 1980, Pub. L. 96-354), section 1102(b) of the Act, section 202 of the Unfunded Mandates Reform Act of 1995 (UMRA, March 22, 1995; Pub. L. 104-4), Executive Order 13132 on Federalism (August 4, 1999), and the Congressional Review Act (5 U.S.C. 804(2)).
Executive Orders 12866 and 13563 direct agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). Executive Order 14094, entitled “Modernizing Regulatory Review”, amends section 3(f)(1) of Executive Order 12866 (Regulatory Planning and Review). The amended section 3(f) of Executive Order 12866 defines a “significant regulatory action” as an action that is likely to result in a rule: (1) having an annual effect on the economy of $200 million or more in any 1 year (adjusted every 3 years by the Administrator of Office of Information and Regulatory Affairs (OIRA) for changes in gross domestic product), or adversely affect in a material way the economy, a sector of the economy, productivity, competition, jobs, the environment, public health or safety, or State, local, territorial, or tribal governments or communities; (2) creating a serious inconsistency or otherwise interfering with an action taken or planned by another agency; (3) materially altering the budgetary impacts of entitlement grants, user fees, or loan programs or the rights and obligations of recipients thereof; or (4) raise legal or policy issues for which centralized review would meaningfully further the President's priorities or the principles set forth in this Executive order, as specifically authorized in a timely manner by the Administrator of OIRA in each case.
A RIA must be prepared for major rules with significant regulatory action/s and/or with significant effects as per section 3(f)(1) ($200 million or more in any 1 year). Based on our estimates, OMB's Office of Information and Regulatory Affairs has determined this rulemaking is significant per section 3(f)(1) as measured by the $200 million or more in any 1 year, and hence also a major rule under subtitle E of the Small Business Regulatory Enforcement Fairness Act of 1996 (also known as the Congressional Review Act). Accordingly, we have prepared a RIA that to the best of our ability presents the costs and benefits of the rulemaking. Therefore, OMB has reviewed the proposed regulations, and the Departments have provided the following assessment of their impact.
3. Overall Impacts
This rule updates the SNF PPS rates contained in the SNF PPS final rule for FY 2024 (88 FR 53200). We estimate that the aggregate impact will be an increase of approximately $1.4 billion (4.2 percent) in Part A payments to SNFs in FY 2025. This reflects a $1.4 billion (4.2 percent) increase from the update to the payment rates. We noted in the proposed rule that these impact numbers do not incorporate the SNF VBP Program reductions that we estimate would total $187.69 million in FY 2025. We note that events may occur to limit the scope or accuracy of our impact analysis, as this analysis is future-oriented, and thus, very susceptible to forecasting errors due to events that may occur within the assessed impact time period.
In accordance with sections 1888(e)(4)(E) and (e)(5) of the Act and implementing regulations at § 413.337(d), we are updating the FY 2024 payment rates by a factor equal to the market basket percentage increase adjusted for the forecast error adjustment and reduced by the productivity adjustment to determine the payment rates for FY 2025. The impact to Medicare is included in the total column of Table 39. The annual update in this rule applies to SNF PPS payments in FY 2025. Accordingly, the analysis of the impact of the annual update that follows only describes the impact of this single year. Furthermore, in accordance with the requirements of the Act, we will publish a rule or notice for each subsequent FY that will provide for an update to the payment rates and include an associated impact analysis.
4. Detailed Economic Analysis
The FY 2025 SNF PPS payment impacts appear in Table 39. Using the most recently available claims data, in this case FY 2023 we apply the current FY 2024 case-mix indices (CMIs), wage index and labor-related share value to the number of payment days to simulate FY 2024 payments. Then, using the same FY 2023 claims data, we apply the FY 2025 CMIs, wage index and labor-related share value to simulate FY 2025 payments. We tabulate the resulting payments according to the classifications in Table 39 (for example, facility type, geographic region, facility ownership), and compare the simulated FY 2024 payments to the simulated FY 2025 payments to determine the overall impact. The breakdown of the various categories of data in Table 39 is as follows:
- The first column shows the breakdown of all SNFs by urban or rural status, hospital-based or freestanding status, census region, and ownership.
- The first row of figures describes the estimated effects of the various changes contained in this final rule on all facilities. The next six rows show the effects on facilities split by hospital-based, freestanding, urban, and rural categories. The next nineteen rows show the effects on facilities by urban versus rural status by census region. The last three rows show the effects on facilities by ownership (that is, government, profit, and non-profit status).
- The second column shows the number of facilities in the impact database.
- The third column shows the effect of the update to the SNF PPS wage index due to adopting the updated census data and revised CBSAs in OMB Bulletin 23-01. This represents the effect of only the adoption of the revised CBSAs, independent of the effect of the annual update to the wage index.
- The fourth column shows the effect of the annual update to the wage index, including the updates to the labor related-share discussed in section VI.A of this final rule. This represents the effect of using the most recent wage data available as well as accounts for the 5 percent cap on wage index transitions. The total impact of this change is 0.0 percent; however, there are distributional effects of the change.
- The fifth column shows the effect of all of the changes on the FY 2025 payments. The update of 4.2 percent is constant for all providers and, though not shown individually, is included in the total column. It is projected that aggregate payments will increase by 4.2 percent, assuming facilities do not change their care delivery and billing practices in response.
As illustrated in Table 39, the combined effects of all of the changes vary by specific types of providers and by location. For example, due to changes in this rule, rural providers will experience a 5.1 percent increase in FY 2025 total payments.
5. Impacts for the Skilled Nursing Facility Quality Reporting Program (SNF QRP) for FY 2027
Estimated impacts for the SNF QRP are based on analysis discussed in section XI. of the proposed rule. In accordance with section 1888(e)(6)(A)(i) of the Act, the Secretary must reduce by 2 percentage points the annual payment update applicable to a SNF for a fiscal year if the SNF does not comply with the requirements of the SNF QRP for that fiscal year.
As stated in section VII.C.3. of this final rule, we are finalizing our proposal to adopt four new items as standardized patient assessment data elements under the SDOH category and modify the Transportation item collected as a standardized patient assessment data element under the SDOH category beginning with residents admitted on October 1, 2025, for the FY 2027 SNF QRP. However, we are finalizing a modification to the data specifications of the new and modified SDOH items so that they exclude any SNF residents who, immediately prior to their hospitalization that preceded a new SNF stay, resided in a NF for at least 366 continuous days.
Although the increase in burden for collecting four new SDOH items and the modified Transportation item via the MDS for each resident at admission only will be accounted for in a revised information collection request under OMB control number (0938-1140), we are providing revised impact information as reflected in Table 40. As discussed in section X.A.2. of this final rule, while the net result of these finalized new and modified SDOH items will increase the burden, the burden of the modified Transportation item will decrease slightly as we are finalizing that SNFs will be required to collect this item at admission only, rather than at admission and discharge as is currently required. With 1,766,806 admissions to and 754,287 planned discharges from 15,477 SNFs annually, we estimate an annual burden increase of 30,565.41hours [(1,766,806 5-day PPS assessments × 0.02 hour for the four new SDOH items) minus [(199,856 5-day PPS assessments × 0.005 hour for the modified Transportation item) plus (754,287 planned discharges × 0.005 hour)]], reflecting a reduction of 4,996.41 hours from the estimate in the proposed rule (89 FR 23424). For each SNF, we estimate an annual burden increase of 1.97 hours (30,565.41hours/15,477 SNFs) at an additional cost of $128.98 ($1,996,226.60 total burden/15,477 SNFs).
As stated in section VII.E.3. of this final rule, we also are finalizing our proposal with modification to require SNFs participating in the SNF QRP to participate in a validation process that will apply to data submitted using the MDS and SNF Medicare fee-for-service claims. Specifically, we are finalizing our proposal with modification to adopt a validation process for the SNF QRP, similar to the process that we adopted for the SNF VBP, beginning with the FY 2027 SNF QRP. This validation process is in accordance with section 111(a)(4) of Division CC of the Consolidated Appropriations Act, 2021 (Pub. L. 116-260) which requires that the measures and data submitted under the SNF QRP Program (section 1888(e)(6) of the Act) be subject to a validation process.
In section VII.E.3(a). of this final rule, we are finalizing our proposal to require SNFs to participate in a validation process for assessment-based measures beginning with the FY 2027 SNF QRP with two modifications. First, as discussed in section VII.E.3.(a) of this final rule, we are finalizing that our validation contractor will select, on an annual basis, up to 1,500 SNFs that submit at least one MDS record in the FY 2 years prior, rather than the CY 3 years prior, to the applicable FY SNF QRP. We are also finalizing regulation text at § 413.360(g)(1)(i) that reflects this new policy. Second, we are modifying the regulation text at § 413.360(g)(1)(iii) to correct a minor technical error, so it properly cross-references paragraph (g)(1) instead of paragraph (g)(2). Our validation contractor will select, on an annual basis, up to 1,500 SNFs and request that each SNF selected for the validation process submit up to 10 medical records. Although the increase in burden will be accounted for in a new information collection request, we are providing impact information. We estimated the burden per selected SNF will range from 3 hours up to 7.5 hours for SNFs utilizing electronic health records and 5 hours up to 12.5 hours for SNFs who do not utilize electronic health records.
We also anticipated that a sample of 10 medical records will consist of SNF stays that vary in length of stay. We estimated the length of stay for each of the selected medical records could range from 1 day up to or exceeding 366 days. We also estimated that approximately 85 percent of nursing homes utilize some form of electronic health records (EHR),[116] and will not incur the costs of printing and shipping records. However, selected SNFs who do not utilize EHRs may incur printing and shipping costs if they are unable to submit the records via an electronic portal, and we estimate the cost to print and ship a sample of up to 10 records will range between $842.67 up to $4,114.35. Therefore, depending on the length of stay of the sample and whether the selected SNF uses an EHR, we estimated the total cost to submit medical records will range between $335,699.85 and $477,368.10 for all 1,500 selected SNFs and $263.29 [$335,699.85/(1,500 × 0.85 SNFs)] and $2,121.64 [$477,368.10/(1,500 × 0.15 SNFs)] per selected SNF. On average, we estimated the total cost will increase by $813,067.95 for all 1,500 selected SNFs [[($263.29 × (1,500 × 0.85)] plus [$2,121.64 × (1,500 × 0.15)]] and $542.05 per selected SNF ($813,067.95/1,500 SNFs) annually.
In section VII.E.3(b). of this final rule, we are finalizing our proposal to require SNFs to participate in a validation process for Medicare fee-for-service claims-based measures beginning with the FY 2027 SNF QRP.
We invited public comments on the overall impact of the SNF QRP proposals for FY 2027 displayed in Table 40.
We have summarized the comments we received in section VII of this final rule and provided responses. After careful consideration of the public comments we received, we are finalizing our proposal with modification as stated above.
6. Impacts for the Minimum Data Set Beginning October 1, 2025
As stated in section X.A.3. of the proposed rule and this final rule, we are removing MDS items that are not needed for case-mix adjusting the SNF per diem payment for PDPM but were not accounted for in the FY 2019 SNF PPS final rule (83 FR 39165 through 39265). We are providing impact information here and in Table 41. With 1,966,662 admissions to 15,477 SNFs annually, we estimate an annual burden decrease of 216,332.82 hours (1,966,662 admissions × 0.11 hour) and a decrease of $14,128,696.47 (216,332.82 hours × $65.31/hr). For each SNF, we estimated an annual burden decrease of 13.98 hours (216,332.82 hours/15,477 SNFs) for a reduction in cost of $912.88 ($14,128,696.47 total burden/15,477 SNFs).
As noted previously in this section of the final rule, we did not formally propose the changes to the MDS. Rather we used this opportunity to provide SNFs the information collection requirements associated with a change that was not accounted for in the FY 2019 SNF PPS final rule. We received a limited number of comments about this notification, and have summarized the comments we received in section X.A.3 of this final rule with our responses.
After careful consideration of the public comments we received, we are finalizing our intention to remove these items.
7. Impacts for the SNF VBP Program
The estimated impacts of the FY 2025 SNF VBP Program are based on historical data and appear in Table 42. We modeled SNF performance in the Program using SNFRM data from FY 2019 as the baseline period and FY 2023 as the performance period. Additionally, we modeled a logistic exchange function with a payback percentage of 60 percent, as we finalized in the FY 2018 SNF PPS final rule (82 FR 36619 through 36621).
For the FY 2025 program year, we will reduce each SNFs adjusted Federal per diem rate by 2 percent. We will then redistribute 60 percent of that 2 percent withhold to SNFs based on their measure performance. Additionally, in the FY 2023 SNF PPS final rule (87 FR 47585 through 47587), we finalized a case minimum requirement for the SNFRM, as required by section 1888(h)(1)(C)(ii) of the Act. As a result of these provisions, SNFs that do not meet the case minimum specified for the SNFRM for the FY 2025 program year will be excluded from the Program and will receive their full Federal per diem rate for that fiscal year. As previously finalized, this policy will maintain the overall payback percentage at 60 percent for the FY 2025 program year. Based on the 60 percent payback percentage, we estimated that we would redistribute approximately $281.53 million (of the estimated $469.22 million in withheld funds) in value-based incentive payments to SNFs in FY 2025, which means that the SNF VBP Program is estimated to result in approximately $187.69 million in savings to the Medicare Program in FY 2025.
Our detailed analysis of the impacts of the FY 2025 SNF VBP Program is shown in Table 42.
In the FY 2024 SNF PPS final rule (88 FR 53324 through 53325), we adopted a validation process that applies to SNF VBP measures calculated using MDS data beginning with the FY 2027 program year. Specifically, we finalized that, on an annual basis, the validation contractor will randomly select up to 1,500 SNFs for validation and that for each SNF selected, the validation contractor will request up to 10 medical records. This new medical record submission requirement for the purposes of SNF VBP MDS validation would result in new burden on SNFs for the FY 2027 program year. We refer readers to the SNF QRP section at XI.A.5. of this final rule for details on the estimated annual burden increase that would result from this new chart submission requirement. We did not include additional details on burden in this SNF VBP section, to avoid double counting burden with the SNF QRP because the same charts will be utilized for both the SNF QRP and SNF VBP Program. We also note that this burden will be accounted for in the information collection request that has been submitted to OMB for approval.
8. Impacts for Nursing Home Enforcement Revisions
A nursing home certified to participate in either the Medicare program as a SNF and Medicaid program as a NF or in both programs as a dually-certified SNF/NF is expected to be in compliance with all applicable Federal requirements of participation as a condition of receiving payment for services provided to beneficiaries. If a facility is determined to be out of compliance and an enforcement decision is reached to impose a civil monetary penalty (CMP) remedy, the finalized provisions set out in these regulatory revisions will be applied as applicable.
We view the anticipated results of this rule as beneficial to nursing home residents as it incentivizes care quality and resident safety. Specifically, we believe that additional flexibility to impose CMPs will allow us to better tailor the response to facility noncompliance in a way that assures that appropriate resident care occurs as well as lasting facility compliance with participation requirements is achieved. We also recognize that not all of the potential effects of this rule can be anticipated. It is difficult to quantify the full future effect of this rule on facilities' compliance activities or costs. If a facility is in substantial compliance with the participation requirements, there is no basis to use any enforcement remedy. However, should a remedy be indicated as an appropriate enforcement response for noncompliance, several alternative remedies may be considered in addition to or in lieu of a CMP. Since CMP amounts, once that remedy is selected as an appropriate enforcement response, are based on when noncompliance occurred and the level of noncompliance, we are unable to predict the number or amount of CMPs that will be imposed. However, we do expect that the total amount of CMPs imposed will increase as a result of these updates.
In 2022, the number of facilities that had a CMP remedy imposed was 6,149 (40 percent). The average total amount of the CMPs imposed for each facility in 2022 was $17,818. The total dollar amount of per day (PD) CMPs imposed on facilities in 2022 was $187.0 million and the total dollar amount of per instance (PI) CMPs imposed was $41.2 million. Additionally, 45 percent of surveys of facilities in 2022 that had multiple findings of harm to residents and that were imposed a PI CMP as the remedy of choice only received one PI CMP. Under the proposed revisions, we anticipate an increased workload to CMS and States, and increased total CMP amounts to providers when multiple instances of noncompliance resulting in harm or immediate jeopardy (IJ) are cited.
We calculated the additional costs for SNFs and NFs, CMS, and States for the multiple PI policy revision by analyzing the number of surveys in CY2022 that would have had additional PI CMPs imposed by identifying surveys with multiple citations of noncompliance resulting in harm or immediate jeopardy (IJ), but only one PI CMP was imposed, or a PD CMP was imposed (109 surveys). We then multiplied the number of these surveys by the average number of citations resulting in harm or IJ (2.3 citations per survey), and by the average PI CMP amount ($9,959). For the PD and PI on the same survey revision, we calculated the additional CMP amounts for surveys that may qualify for PD and PI CMPs by multiplying the number of surveys with at least 2 citations resulting in harm or IJ and were only imposed a PD CMP (787) by the average number of harm or IJ citations per survey (2.8) and also multiplying by the average PI CMP amount ($9,959). Adding the estimated additional cost to nursing homes for enabling multiple PI CMPs for a survey with the estimated additional cost for enabling PI CMPs to surveys with PD CMPs resulted in a total of approximately $25 million for all nursing homes for CY2022.
We calculated the additional costs for CMS and States by multiplying the average hourly rate of CMS staff ($84.00 per hour) by the average number of hours spent by CMS staff per CMP (0.8 hours per CMP) by the total number of anticipated increased CMPs for surveys that qualify for either multiple PI CMPs (109 surveys × 2.3 average citations resulting in harm or IJ) or surveys that qualify for PD and PI CMPs (787 surveys × 2.8 average citations resulting in harm or IJ). We estimate this will result in a total increased cost to CMS and the States of $164,929 per year. Note: The estimated impact of the third proposed change related to the timing of imposing a CMP is embedded in these amounts, as these estimates are inclusive of any cases where CMS needs to impose a CMP for noncompliance that was previously cited, but no CMP has yet been imposed.
9. Alternatives Considered
As described in this section, we estimate that the aggregate impact of the provisions in this final rule will result in an increase of approximately $1.4 billion (4.2 percent) in Part A payments to SNFs in FY 2025. This reflects a $1.4 billion (4.2 percent) increase from the update to the payment rates.
Section 1888(e) of the Act establishes the SNF PPS for the payment of Medicare SNF services for cost reporting periods beginning on or after July 1, 1998. This section of the statute prescribes a detailed formula for calculating base payment rates under the SNF PPS, and does not provide for the use of any alternative methodology. It specifies that the base year cost data to be used for computing the SNF PPS payment rates must be from FY 1995 (October 1, 1994, through September 30, 1995). In accordance with the statute, we also incorporated a number of elements into the SNF PPS (for example, case-mix classification methodology, a market basket update, a wage index, and the urban and rural distinction used in the development or adjustment of the Federal rates). Further, section 1888(e)(4)(H) of the Act specifically requires us to disseminate the payment rates for each new FY through the Federal Register , and to do so before the August 1 that precedes the start of the new FY; accordingly, we are not pursuing alternatives for this process.
With regard to adopting four new assessment items as standardized patient assessment data elements under the SDOH category and modifying the Transportation standardized patient assessment data element in the SDOH category beginning with the FY 2027 SNF QRP, we believe these new and modified items advance the CMS National Quality Strategy Goals of equity and engagement. We considered the alternative of delaying the collection of these four new assessment items. However, given the fact they will encourage meaningful collaboration between healthcare providers, residents, caregivers, and community-based organizations to address SDOH prior to discharge from the SNF, we believe further delay is unwarranted.
With regard to removing 22 items from the MDS beginning October 1, 2025, we routinely review the MDS for opportunities to simplify data submission requirements. We have identified that these items are no longer used in the calculation of the SNF per diem payment for PDPM but were not accounted for in the FY 2019 SNF PPS final rule (83 FR 39165 through 39265), and therefore no alternatives were considered.
With regard to requiring SNFs participating in the SNF QRP to participate in a validation process beginning with the FY 2027 SNF QRP, we are required to implement a process to satisfy section 1888(h)(12) of the Act (as added by Division CC, section 111(a)(4) of the Consolidated Appropriations Act, 2021 (Pub. L. 116-120)). Because the validation process is statutorily required, no alternatives were considered.
With regard to the updates for the SNF VBP Program, we discussed alternatives considered within those sections. In section VII.E.3. of the proposed rule, we discussed other approaches to incorporating health equity into the Program.
With regard to the updates for the nursing home enforcement program, we discussed alternatives within those sections. In section IX.A. of the proposed rule, we discussed how current regulatory limitations create inequity in the imposition of PD CMPs and the need for additional flexibility to ensure that CMP amounts are more closely aligned with the noncompliance that occurred and are thus effective to encourage facilities to return and sustain compliance.
10. Accounting Statement
As required by OMB Circular A-4 (available online at https://www.whitehouse.gov/wp-content/uploads/2023/11/CircularA-4.pdf), in Tables 43 through 47, we have prepared an accounting statement showing the classification of the expenditures associated with the provisions of the proposed rule for FY 2025. Tables 39 and 43 provide our best estimate of the possible changes in Medicare payments under the SNF PPS as a result of the policies outlined in this final rule, based on the data for 15,477 SNFs in our database. Tables 40, 44, and 45 provide our best estimate of the additional cost to SNFs to submit the data for the SNF QRP as a result of the policies outlined in this final rule. Table 46 provides our best estimate of the possible changes in Medicare payments under the SNF VBP as a result of the policies for this program. Table 47 provides our best estimate of the Nursing Home Enforcement provisions.
11. Conclusion
This rule updates the SNF PPS rates contained in the SNF PPS final rule for FY 2024 (88 FR 53200). Based on the above, we estimate that the overall payments for SNFs under the SNF PPS in FY 2025 are projected to increase by approximately $1.4 billion, or 4.2 percent, compared with those in FY 2024. We estimate that in FY 2025, SNFs in urban and rural areas will experience, on average, a 4.1 percent increase and 5.1 percent increase, respectively, in estimated payments compared with FY 2024. Providers in the rural Middle Atlantic region will experience the largest estimated increase in payments of approximately 7.4 percent. Providers in the urban Outlying region will experience the smallest estimated increase in payments of 1.5 percent.
B. Regulatory Flexibility Act Analysis
The RFA requires agencies to analyze options for regulatory relief of small entities, if a rule has a significant impact on a substantial number of small entities. For purposes of the RFA, small entities include small businesses, non-profit organizations, and small governmental jurisdictions. Most SNFs and most other providers and suppliers are small entities, either by reason of their non-profit status or by having revenues of $30 million or less in any 1 year. We utilized the revenues of individual SNF providers (from recent Medicare Cost Reports) to classify a small business, and not the revenue of a larger firm with which they may be affiliated. As a result, for the purposes of the RFA, we estimate that almost all SNFs are small entities as that term is used in the RFA, according to the Small Business Administration's latest size standards (NAICS 623110), with total revenues of $34 million or less in any 1 year. (For details, see the Small Business Administration's website at https://www.sba.gov/category/navigation-structure/contracting/contracting-officials/eligibility-size-standards.) In addition, approximately 20 percent of SNFs classified as small entities are non-profit organizations. Finally, individuals and States are not included in the definition of a small entity.
This rule updates the SNF PPS rates contained in the SNF PPS final rule for FY 2024 (88 FR 53200). Based on the above, we estimate that the aggregate impact for FY 2025 will be an increase of $1.4 billion in payments to SNFs, resulting from the SNF market basket update to the payment rates. While it is projected in Table 39 that all providers will experience a net increase in payments, we note that some individual providers within the same region or group may experience different impacts on payments than others due to the distributional impact of the FY 2025 wage indexes and the degree of Medicare utilization.
Guidance issued by the Department of Health and Human Services on the proper assessment of the impact on small entities in rulemakings, utilizes a cost or revenue impact of 3 to 5 percent as a significance threshold under the RFA. In their March 2024 Report to Congress (available at https://www.medpac.gov/wp-content/uploads/2024/03/Mar24_Ch6_MedPAC_Report_To_Congress_SEC.pdf), MedPAC states that Medicare covers approximately 10 percent of total patient days in freestanding facilities and 17 percent of facility revenue (March 2024 MedPAC Report to Congress, 168). As indicated in Table 39, the effect on facilities is projected to be an aggregate positive impact of 4.2 percent for FY 2025. As the overall impact on the industry as a whole, and thus on small entities specifically, meets the 3 to 5 percent threshold discussed previously, the Secretary has determined that this final rule will have a significant impact on a substantial number of small entities for FY 2025.
In addition, section 1102(b) of the Act requires us to prepare a regulatory impact analysis if a rule may have a significant impact on the operations of a substantial number of small rural hospitals. This analysis must conform to the provisions of section 604 of the RFA. For purposes of section 1102(b) of the Act, we define a small rural hospital as a hospital that is located outside of an MSA and has fewer than 100 beds. This final rule will affect small rural hospitals that: (1) furnish SNF services under a swing-bed agreement or (2) have a hospital-based SNF. We anticipate that the impact on small rural hospitals will be similar to the impact on SNF providers overall. Moreover, as noted in previous SNF PPS final rules (most recently, the one for FY 2024 (88 FR 53200)), the category of small rural hospitals is included within the analysis of the impact of the proposed rule on small entities in general. As indicated in Table 39, the effect on facilities for FY 2025 is projected to be an aggregate positive impact of 4.2 percent. As the overall impact on the industry as a whole meets the 3 to 5 percent threshold discussed previously, the Secretary has determined that this final rule will have a significant impact on a substantial number of small rural hospitals for FY 2025.
C. Unfunded Mandates Reform Act Analysis
Section 202 of the Unfunded Mandates Reform Act of 1995 also requires that agencies assess anticipated costs and benefits before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995 dollars, updated annually for inflation. In 2024, that threshold is approximately $183 million. This final rule will impose no mandates on State, local, or Tribal governments or on the private sector.
D. Federalism Analysis
Executive Order 13132 establishes certain requirements that an agency must meet when it issues a proposed rule (and subsequent final rule) that imposes substantial direct requirement costs on State and local governments, preempts State law, or otherwise has federalism implications. This final rule will have no substantial direct effect on State and local governments, preempt State law, or otherwise have federalism implications.
E. Regulatory Review Costs
If regulations impose administrative costs on private entities, such as the time needed to read and interpret this final rule, we should estimate the cost associated with regulatory review. Due to the uncertainty involved with accurately quantifying the number of entities that will review the rule, we assume that the total number of unique commenters on this year's proposed rule will be the number of reviewers of this year's final rule. We acknowledge that this assumption may understate or overstate the costs of reviewing this rule. It is possible that not all commenters reviewed this year's proposed rule in detail, and it is also possible that some reviewers chose not to comment on the proposed rule. For these reasons, we believe that the number of commenters on this year's proposed rule is a fair estimate of the number of reviewers of this final rule.
We also recognize that different types of entities are in many cases affected by mutually exclusive sections of this final rule, and therefore, for the purposes of our estimate we assume that each reviewer reads approximately 50 percent of the rule.
The mean wage rate for medical and health service manages (SOC 11-9111) in BLS Occupational Employment and Wage Statistics (OEWS) is $64.64, assuming benefits plus other overhead costs equal 100 percent of wage rate, we estimate that the cost of reviewing this rule is $129.28 per hour, including overhead and fringe benefits https://www.bls.gov/oes/current/oes_nat.htm. Assuming an average reading speed, we estimate that it will take approximately 4 hours for the staff to review half of this final rule. For each SNF that reviews the rule, the estimated cost is $517.12 (4 hours × $129.28). Therefore, we estimate that the total cost of reviewing this regulation is $227,015.68 ($517.12 × 439 reviewers).
In accordance with the provisions of Executive Order 12866, this final rule is reviewed by the Office of Management and Budget.
Chiquita Brooks-LaSure, Administrator of the Centers for Medicare & Medicaid Services, approved this document on July 24, 2024.
List of Subjects
42 CFR Part 413
- Diseases
- Health facilities
- Medicare
- Puerto Rico
- Reporting and recordkeeping requirements
42 CFR Part 488
- Administrative practice and procedure
- Health facilities
- Health professions
- Medicare
- Reporting and recordkeeping requirements
For the reasons set forth in the preamble, the Centers for Medicare & Medicaid Services amends 42 CFR chapter IV as set forth below:
PART 413—PRINCIPLES OF REASONABLE COST REIMBURSEMENT; PAYMENT FOR END-STAGE RENAL DISEASE SERVICES; PROSPECTIVELY DETERMINED PAYMENT RATES FOR SKILLED NURSING FACILITIES; PAYMENT FOR ACUTE KIDNEY INJURY DIALYSIS
1. The authority citation for part 413 continues to read as follows:
2. Section 413.337 is amended by revising paragraph (f) to read as follows:
Methodology for calculating the prospective payment rates.* * * * *(f) Adjustments to payment rates under the SNF Value-Based Purchasing Program. Beginning with payment for services furnished on October 1, 2018, the adjusted Federal per diem rate (as defined in § 413.338(a)) otherwise applicable to a SNF for the fiscal year is reduced by the applicable percent (as defined in § 413.338(a)). The resulting amount is then adjusted by the value-based incentive payment amount (as defined in § 413.338(a)) based on the SNF performance score calculated for the SNF for that fiscal year under § 413.338.
3. Section 413.338 is amended—
a. In paragraph (a) by—
i. Revising the definitions of “Health equity adjustment (HEA) bonus points” and “Measure performance scaler”;
ii. Removing the definition of “Performance score”;
iii. Adding the definition of “SNF performance score” in alphabetical order; and
iv. Revising the definitions of “SNF readmission measure”, “Top tier performing SNF”, and “Underserved multiplier”;
b. Removing paragraphs (d)(4) through (6);
c. Redesignating paragraphs (f)(1) through (4) as paragraphs (f)(2) through (5);
d. Adding a new paragraph (f)(1) and revising newly redesignated paragraphs (f)(2) and (3);
e. In newly redesignated paragraph (f)(4) introductory text by removing the reference “paragraphs (f)(1) and (2)” and adding in its place the reference “paragraphs (f)(2) and (3)”;
f. Revising paragraph (j)(3); and
g. Adding paragraphs (l), (m), and (n).
The revisions and additions read as follows:
Skilled nursing facility value-based purchasing program.(a) * * *
Health equity adjustment (HEA) bonus points means the points that a SNF can earn for a fiscal year based on its performance and proportion of SNF residents who are members of the underserved population.
* * * * *Measure performance scaler means, for a fiscal year, the sum of the points assigned to a SNF for each measure on which the SNF is a top tier performing SNF.
* * * * *SNF performance score means the numeric score ranging from 0 to 100 awarded to each SNF based on its performance under the SNF VBP Program for a fiscal year.
SNF readmission measure means, prior to October 1, 2027, the SNF 30-Day All-Cause Readmission Measure (SNFRM) specified under section 1888(g)(1) of the Social Security Act. Beginning October 1, 2027, the term SNF readmission measure means the SNF Within-Stay Potentially Preventable Readmission (SNF WS PPR) Measure specified under section 1888(g)(2) of the Social Security Act.
* * * * *Top tier performing SNF means a SNF whose performance on a measure during the applicable fiscal year meets or exceeds the 66.67th percentile of SNF performance on the measure during the same fiscal year.
Underserved multiplier means the mathematical result of applying a logistic function to the number of SNF residents who are members of the underserved population out of the SNF's total Medicare population, as identified from the SNF's Part A claims, during the performance period that applies to the 1-year measures for the applicable fiscal year.
* * * * *(f) * * *
(1) CMS will provide quarterly confidential feedback reports to SNFs on their performance on each measure specified for the fiscal year. Beginning with the baseline period and performance period quality measure quarterly reports issued on or after October 1, 2021, CMS calculates the measure rates included in those reports using data that are current as of a specified date as follows:
(i) For the SNFRM, the specified date is 3 months after the last index SNF admission in the applicable baseline period or performance period.
(ii) For the Skilled Nursing Facility Healthcare Associated Infections Requiring Hospitalization (“SNF HAI”), Discharge to Community—Post-Acute Care Measure for Skilled Nursing Facilities (“DTC PAC SNF”), and Skilled Nursing Facility Within-Stay Potentially Preventable Readmissions (“SNF WS PPR”) measure, the specified date is 3 months after the last SNF discharge in the applicable baseline period or performance period.
(iii) For the Number of Hospitalizations per 1,000 Long Stay Residents (“Long Stay Hospitalization”) measure, the specified date is 3 months after the last day of the final quarter of the applicable baseline period or performance period.
(iv) For the Total Nursing Hours per Resident Day Staffing (“Total Nurse Staffing”) measure and the Total Nursing Staff Turnover (“Nursing Staff Turnover”) measure, the specified date is 45 days after the last day of each quarter of the applicable baseline period or performance period.
(v) For the Discharge Function Score for SNFs (“DC Function measure”) and Percent of Residents Experiencing One of More Falls with Major Injury (Long Stay) (“Falls with Major Injury (Long Stay)”) measure, the specified date is the February 15th that is approximately 4.5 months after the last day of the applicable baseline period or performance period.
(2) Beginning with the baseline period and performance period quality measure quarterly reports issued on or after October 1, 2021, which contain the baseline period and performance period measure rates, respectively, SNFs will have 30 days following the date CMS provides in each of these reports to review and submit corrections to the measure rate calculations contained in that report. The underlying data used to calculate the measure rates are not subject to review and correction under this paragraph (f)(2). Any such correction requests must include:
(i) The SNF's CMS Certification Number (CCN);
(ii) The SNF's name;
(iii) The correction requested; and
(iv) The reason for requesting the correction, including any available evidence to support the request.
(3) Beginning not later than 60 days prior to each fiscal year, CMS will provide reports to SNFs on their performance under the SNF VBP Program for a fiscal year. SNFs will have the opportunity to review and submit corrections to their SNF performance scores and ranking contained in these reports for 30 days following the date that CMS provides the reports. Any such correction requests must include:
(i) The SNF's CMS Certification Number (CCN);
(ii) The SNF's name;
(iii) The correction requested; and
(iv) The reason for requesting the correction, including any available evidence to support the request.
* * * * *(j) * * *
(3) Beginning October 1, 2026, for all measures that are calculated using Minimum Data Set (MDS) information, CMS will validate the accuracy of this information. CMS will request medical records as follows:
(i) On an annual basis, a CMS contractor will randomly select up to 1,500 SNFs for validation. A SNF is eligible for selection for a year if the SNF submitted at least one MDS record in the calendar year that is 3 years prior to the applicable fiscal year or was included in the SNF VBP Program in the year prior to the applicable fiscal year.
(ii) For each SNF selected under paragraph (j)(3)(i) of this section, the CMS contractor will request in writing up to 10 medical records.
(iii) A SNF that receives a request for medical records under paragraph (j)(3)(ii) of this section must submit a digital or paper copy of each of the requested medical records within 45 days of the date of the request as documented on the request.
* * * * *(l) Measure selection, retention, and removal policy. (1) The SNF VBP measure set for each fiscal year includes the SNF readmission measure CMS has specified under section 1888(g) of the Social Security Act for application in the SNF VBP Program.
(2) Beginning with FY 2026, the SNF VBP measure set for each fiscal year may include up to nine additional measures specified by CMS. Each of these measures remains in the measure set unless CMS removes or replaces it based on one or more of the following factors:
(i) SNF performance on the measure is so high and unvarying that meaningful distinctions and improvements in performance can no longer be made.
(ii) Performance or improvement on a measure do not result in better resident outcomes.
(iii) A measure no longer aligns with current clinical guidelines or practices.
(iv) A more broadly applicable measure for the particular topic is available.
(v) A measure that is more proximal in time to the desired resident outcomes for the particular topic is available.
(vi) A measure that is more strongly associated with the desired resident outcomes for the particular topic is available.
(vii) The collection or public reporting of a measure leads to negative unintended consequences other than resident harm.
(viii) The costs associated with a measure outweigh the benefit of its continued use in the Program.
(3) Upon a determination by CMS that the continued requirement for SNFs to submit data on a measure specified under paragraph (l)(2) of this section raises specific resident safety concerns, CMS may elect to immediately remove the measure from the SNF VBP Program. Upon removal of the measure, CMS will provide notice to SNFs and the public, along with a statement of the specific patient safety concern that would be raised if SNFs continued to submit data on the measure. CMS will also provide notice of the removal in the Federal Register .
(4) CMS uses rulemaking to make substantive updates to the specifications of measures used in the SNF VBP Program. CMS makes technical measure specification updates in a sub-regulatory manner and informs SNFs of measure specification updates through postings on the CMS website, listservs, and other educational outreach efforts to SNFs.
(m) Extraordinary circumstances exception policy. (1) A SNF may request and CMS may grant exceptions to the SNF Value-Based Purchasing Program's requirements under this section for one or more calendar months when there are certain extraordinary circumstances beyond the control of the SNF.
(2) A SNF may request an exception within 90 days of the date that the extraordinary circumstances occurred. Prior to FY 2025, the request must be submitted in the form and manner specified by CMS on the SNF VBP website at https://www.cms.gov/Medicare/Quality/Nursing-Home-Improvement/Value-Based-Purchasing/Extraordinary-Circumstance-Exception and include a completed Extraordinary Circumstances Request form (available on https://qualitynet.cms.gov/) and any available evidence of the impact of the extraordinary circumstances on the care that the SNF furnished to patients including, but not limited to, photographs and media articles. Beginning with FY 2025, a SNF may request an extraordinary circumstances exception by sending an email with the subject line “SNF VBP Extraordinary Circumstances Exception Request” to the SNF VBP Program Help Desk with the following information:
(i) The SNF's CMS Certification Number (CCN);
(ii) The SNF's business name and business address;
(iii) Contact information for the SNF's chief executive officer (CEO) or CEO-designated personnel, including all applicable names, email addresses, telephone numbers, and the SNF's physical mailing address (which cannot be a P.O. Box);
(iv) A description of the event, including the dates and duration of the extraordinary circumstance;
(v) Available evidence of the impact of the extraordinary circumstance on the care the SNF provided to its residents or the SNF's ability to report SNF VBP data, including, but not limited to, photographs, media articles, and any other materials that would aid CMS in determining whether to grant the exception; and
(vi) A date proposed by the SNF for when it will again be able to fully comply with the SNF VBP Program's requirements and a justification for the proposed date.
(3) Except as provided in paragraph (m)(4) of this section, CMS will not consider an exception request unless the SNF requesting such exception has complied fully with the requirements in paragraph (m)(2) of this section.
(4) CMS may grant exceptions to SNFs without a request if it determines that an extraordinary circumstance affected an entire region or locale.
(5) CMS will calculate a SNF performance score for a fiscal year for a SNF for which it has granted an exception request that does not include its performance on a quality measure during the calendar months affected by the extraordinary circumstance.
(n) SNF VBP performance standards. (1) CMS announces the performance standards for each measure no later than 60 days prior to the start of the performance period that applies to the measure for the fiscal year.
(2) Beginning with FY 2021, if CMS discovers an error in the performance standard calculations subsequent to publishing their numerical values for a fiscal year, CMS will update the numerical values to correct the error. If CMS subsequently discovers one or more other errors with respect to the fiscal year, CMS will not further update the numerical values for that fiscal year.
(3) Beginning with FY 2025, CMS may update the numerical values of the performance standards for a measure if, between the time that CMS announced the performance standards for the measure for that fiscal year and the time that CMS calculates SNF performance on the measure at the conclusion of the performance period for that measure for that fiscal year, CMS has made technical updates to the specifications for the measure that affect the measure rate calculations.
4. Section 413.360 is amended by—
a. Revising paragraph (f)(1) introductory text;
b. Adding paragraph (f)(1)(iv);
c. Revising paragraph (f)(3); and
d. Adding paragraph (g).
The additions and revision read as follows:
Requirements under the Skilled Nursing Facility (SNF) Quality Reporting Program (QRP).* * * * *(f) * * *
(1) SNFs must meet or exceed the following data completeness thresholds with respect to a program year:
* * * * *(iv) If selected for the data validation process under paragraph (g) of this section, the threshold set at 100 percent submission of medical charts.
* * * * *(3) A SNF must meet or exceed each applicable threshold described in paragraph (f)(1) of this section to avoid receiving the applicable penalty for failure to report quality data set forth in § 413.337(d)(4).
(g) Data validation process. (1) Beginning with the FY 2027 payment year: for all measures that are calculated using Minimum Data Set (MDS) information, CMS will validate the accuracy of this information. The process by which CMS will request medical records and by which SNFs must submit the requested medical records is as follows:
(i) On an annual basis, a CMS contractor will select up to 1,500 SNFs for validation. A SNF is eligible for selection for a year if it submitted at least one MDS record to CMS in the fiscal year that is 2 years prior to the applicable program year, and if the SNF has been randomly selected for a periodic audit for the same year under § 413.338.
(ii) For each SNF selected under this paragraph (g)(1), the CMS contractor will request up to 10 medical records. Each SNF selected will only be required to submit records once in a fiscal year, for a maximum of 10 records for each SNF selected. Each requested medical record must be the same medical record that has been requested for submission by the SNF for the same year under § 413.338. CMS will submit its request in writing to the selected SNF.
(iii) A SNF that receives a request for medical records under this paragraph (g)(1) must submit a digital or paper copy of each of the requested medical records within 45 days of the date of the request.
(2) Beginning with the FY 2027 payment year: the information reported through claims for all claims-based measures are validated for accuracy by Medicare Administrative Contractors (MACs).
PART 488—SURVEY, CERTIFICATION, AND ENFORCEMENT PROCEDURES
5. The authority citation for part 488 continues to read as follows:
6. Section 488.401 is amended by adding the definition of “Instance or instances of noncompliance” in alphabetical order to read as follows:
Definitions.* * * * *Instance or instances of noncompliance means a factual and temporal occurrence(s) when a facility is not in substantial compliance with the requirements for participation. Each instance of noncompliance is sufficient to constitute a deficiency and a deficiency may comprise of multiple instances of noncompliance.
* * * * *7. Section 488.408 is amended by revising paragraph (e)(2)(ii) to read as follows:
Selection of remedies.* * * * *(e) * * *
(2) * * *
(ii) For each instance of noncompliance, CMS and the State may impose a civil money penalty of $3,050-$10,000 (as adjusted annually under 45 CFR part 102) per day, $1,000-$10,000 (as adjusted annually under 45 CFR part 102) per instance of noncompliance, or both, in addition to imposing the remedies specified in paragraph (e)(2)(i) of this section. For multiple instances of noncompliance, CMS may impose any combination of per instance or per day civil money penalties for each instance within the same survey. The aggregate civil money penalty amount may not exceed $10,000 (as adjusted annually under 45 CFR part 102) for each day of noncompliance.
* * * * *8. Section 488.430 is revised to read as follows:
Civil money penalties: Basis for imposing penalty.(a) CMS or the State may impose a civil money penalty for the number of days a facility is not in substantial compliance with one or more participation requirements or for each instance that a facility is not in substantial compliance, or both, regardless of whether or not the deficiencies constitute immediate jeopardy. When a survey contains multiple instances of noncompliance, CMS or the State may impose any combination of per instance or per day civil money penalties for each instance of noncompliance within the same survey.
(b) CMS or the State may impose a civil money penalty for the number of days or instances of previously cited noncompliance, including the number of days of immediate jeopardy, since the last three standard surveys.
9. Section 488.434 is amended by revising paragraphs (a)(2)(iii) and (v) to read as follows:
Civil money penalties: Notice of penalty.(a) * * *
(2) * * *
(iii) Either the amount of penalty per day of noncompliance or the amount of the penalty per instance of noncompliance or both;
* * * * *(v) The date(s) of the instance(s) of noncompliance or the date on which the penalty begins to accrue;
* * * * *10. Section 488.440 is amended by revising paragraph (a)(2) to read as follows:
Civil money penalties: Effective date and duration of penalty.(a) * * *
(2) A civil money penalty for each instance of noncompliance is imposed in a specific amount per instance.
* * * * *Xavier Becerra,
Secretary, Department of Health and Human Services.
Footnotes
2. Items may also be referred to as “data elements.”
Back to Citation3. As noted in section VI.C.3 of the proposed rule and section VII.C.3 of this final rule, hospitals are required to report whether they have screened patients for five standardized SDOH categories: housing instability, food insecurity, utility difficulties, transportation needs, and interpersonal safety.
Back to Citation4. FY 2020 SNF PPS final rule (84 FR 38805).
Back to Citation5. World Health Organization. Social determinants of health. Available at https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1.
Back to Citation6. Using Z Codes: The Social Determinants of Health (SDOH). Data Journey to Better Outcomes.
7. Improving the Collection of Social Determinants of Health (SDOH) Data with ICD-10-CM Z Codes. https://www.cms.gov/files/document/cms-2023-omh-z-code-resource.pdf.
8. CMS.gov. Measures Management System (MMS). CMS Focus on Health Equity. Health Equity Terminology and Quality Measures. https://mmshub.cms.gov/about-quality/quality-at-CMS/goals/cms-focus-on-health-equity/health-equity-terminology.
Back to Citation9. Centers for Disease Control and Prevention. Social Determinants of Health (SDOH) and PLACES Data.
10. “U.S. Playbook To Address Social Determinants Of Health” from the White House Office Of Science And Technology Policy (November 2023).
Back to Citation11. These SDOH data are also collected for purposes outlined in section 2(d)(2)(B) of the Improving Medicare Post-Acute Care Transitions Act (IMPACT Act). For a detailed discussion on SDOH data collection under section 2(d)(2)(B) of the IMPACT Act, see the FY 2020 SNF PPS final rule (84 FR 38805 through 38817).
Back to Citation12. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for Social Risk Factors in Medicare Payment: Identifying Social Risk Factors. Washington, DC: The National Academies Press. https://doi.org/10.17226/21858.
Back to Citation13. National Academies of Sciences, Engineering, and Medicine. 2020. Leading Health Indicators 2030: Advancing Health, Equity, and Well-Being. Washington, DC: The National Academies Press. https://doi.org/10.17226/25682.
Back to Citation14. Centers for Medicare & Medicaid Services. “A Guide to Using the Accountable Health Communities Health-Related Social Needs Screening Tool: Promising Practices and Key Insights.” August 2022. Available at https://www.cms.gov/priorities/innovation/media/document/ahcm-screeningtool-companion.
Back to Citation15. Berkowitz, S.A., T.P. Baggett, and S.T. Edwards, “Addressing Health-Related Social Needs: Value-Based Care or Values-Based Care?” Journal of General Internal Medicine, vol. 34, no. 9, 2019, pp. 1916-1918, https://doi.org/10.1007/s11606-019-05087-3.
Back to Citation16. Hugh Alderwick and Laura M. Gottlieb, “Meanings and Misunderstandings: A Social Determinants of Health Lexicon for Health Care Systems: Milbank Quarterly,” Milbank Memorial Fund, November 18, 2019, https://www.milbank.org/quarterly/articles/meanings-and-misunderstandings-a-social-determinants-of-health-lexicon-for-health-care-systems/.
Back to Citation17. American Hospital Association (2020). Health Equity, Diversity & Inclusion Measures for Hospitals and Health System Dashboards. December 2020. Accessed: January 18, 2022. Available at https://ifdhe.aha.org/system/files/media/file/2020/12/ifdhe_inclusion_dashboard.pdf.
Back to Citation18. In October 2023, we released two new annual Health Equity Confidential Feedback Reports to SNFs: The Discharge to Community (DTC) Health Equity Confidential Feedback Report and the Medicare Spending Per Beneficiary (MSPB) Health Equity Confidential Feedback Report. The PAC Health Equity Confidential Feedback Reports stratified the DTC and MSPB measures by dual-enrollment status and race/ethnicity. For more information on the Health Equity Confidential Feedback Reports, please refer to the Education and Outreach materials available on the SNF QRP Training web page at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/Skilled-Nursing-Facility-Quality-Reporting-Program/SNF-Quality-Reporting-Program-Training.
Back to Citation19. Brooks-LaSure, C. (2021). My First 100 Days and Where We Go from Here: A Strategic Vision for CMS. Centers for Medicare & Medicaid. Available at https://www.cms.gov/blog/my-first-100-days-and-where-we-go-here-strategic-vision-cms.
Back to Citation20. The Biden-Harris Administration's strategic approach to addressing health related social needs can be found in The U.S. Playbook to Address Social Determinants of Health (SDOH) (2023): https://www.whitehouse.gov/wp-content/uploads/2023/11/SDOH-Playbook-3.pdf.
Back to Citation21. The AHC Model was a 5-year demonstration project run by the Centers for Medicare & Medicaid Innovation between May 1, 2017 and April 30, 2023. For more information go to https://www.cms.gov/priorities/innovation/innovation-models/ahcm.
Back to Citation22. More information about the AHC HRSN Screening Tool is available on the website at https://innovation.cms.gov/Files/worksheets/ahcm-screeningtool.pdf.
Back to Citation23. Centers for Medicare & Medicaid Services, FY2023 IPPS/LTCH PPS final rule (87 FR 49202 through 49215).
Back to Citation24. Centers for Medicare & Medicaid Services, FY2024 Inpatient Psychiatric Prospective Payment System—Rate Update (88 FR 51107 through 51121).
Back to Citation25. Office of Disease Prevention and Health Promotion. (n.d.). Healthy People 2030 | Priority Areas: Social Determinants of Health. Retrieved from U.S. Department of Health and Human Services: https://health.gov/healthypeople/priority-areas/social-determinants-health.
26. Healthy People 2030 is a long-term, evidence-based effort led by the U.S. Department of Health and Human Services (HHS) that aims to identify nationwide health improvement priorities and improve the health of all Americans.
Back to Citation27. Kushel, M.B., Gupta, R., Gee, L., & Haas, J.S. (2006). Housing instability and food insecurity as barriers to health care among low-income Americans. Journal of General Internal Medicine, 21 (1), 71-77. doi: 10.1111/j.1525-1497.2005.00278.x.
Back to Citation28. Homelessness is defined as “lacking a regular nighttime residence or having a primary nighttime residence that is a temporary shelter or other place not designed for sleeping.” Crowley, S. (2003). The affordable housing crisis: Residential mobility of poor families and school mobility of poor children. Journal of Negro Education, 72(1), 22-38. https://doi.org/10.2307/3211288.
Back to Citation29. The 2023 Annual Homeless Assessment Report (AHAR) to Congress. The U.S. Department of Housing and Urban Development 2023. https://www.huduser.gov/portal/sites/default/files/pdf/2023-AHAR-Part-1.pdf.
Back to Citation30. Baggett, T.P., Hwang, S.W., O'Connell, J.J., Porneala, B.C., Stringfellow, E.J., Orav, E.J., Singer, D.E., & Rigotti, N.A. (2013). Mortality among homeless adults in Boston: Shifts in causes of death over a 15-year period. JAMA Internal Medicine, 173(3), 189-195. https://doi.org/10.1001/jamainternmed.2013.1604. Schanzer, B., Dominguez, B., Shrout, P.E., & Caton, C.L. (2007). Homelessness, health status, and health care use. American Journal of Public Health, 97(3), 464-469. doi: https://doi.org/10.2105/ajph.2005.076190.
Back to Citation31. U.S. Department of Health & Human Services (HHS), Call to Action, “Addressing Health Related Social Needs in Communities Across the Nation.” November 2023. https://aspe.hhs.gov/sites/default/files/documents/3e2f6140d0087435cc6832bf8cf32618/hhs-call-to-action-health-related-social-needs.pdf.
Back to Citation32. Henderson, K.A., Manian, N., Rog, D.J., Robison, E., Jorge, E., AlAbdulmunem, M. “Addressing Homelessness Among Older Adults” (Final Report). Washington, DC: Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. October 26, 2023.
Back to Citation33. More information about the AHC HRSN Screening Tool is available on the website at https://innovation.cms.gov/Files/worksheets/ahcm-screeningtool.pdf.
34. The AHC HRSN Screening Tool Living Situation item includes two questions. In an effort to limit SNF burden, we only proposed the first question.
Back to Citation35. National Association of Community Health Centers and Partners, National Association of Community Health Centers, Association of Asian Pacific Community Health Organizations, Association OPC, Institute for Alternative Futures. “PRAPARE.” 2017. https://prapare.org/the-prapare-screening-tool /.
Back to Citation36. U.S. Department of Agriculture, Economic Research Service (n.d.). Definitions of food security. Retrieved March 10, 2022, from https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-u-s/definitions-of-food-security/.
Back to Citation37. Hernandez, D.C., Reesor, L.M., & Murillo, R. (2017). Food insecurity and adult overweight/obesity: Gender and race/ethnic disparities. Appetite, 117, 373-378.
Back to Citation38. Banerjee, S., Radak, T., Khubchandani, J., & Dunn, P. (2021). Food Insecurity and Mortality in American Adults: Results From the NHANES-Linked Mortality Study. Health promotion practice, 22(2), 204-214. https://doi.org/10.1177/1524839920945927
Back to Citation39. National Center for Health Statistics (2022, September 6). Exercise or Physical Activity. Retrieved from Centers for Disease Control and Prevention: https://www.cdc.gov/nchs/fastats/exercise.htm.
Back to Citation40. Ziliak, J.P., & Gundersen, C. (2019). The State of Senior Hunger in America 2017: An Annual Report. Prepared for Feeding America. Available at https://www.feedingamerica.org/research/senior-hunger-research/senior.
Back to Citation41. The Malnutrition Quality Collaborative (2020). National Blueprint: Achieving Quality Malnutrition Care for Older Adults, 2020 Update. Washington, DC: Avalere Health and Defeat Malnutrition Today. Available at https://defeatmalnutrition.today/advocacy/blueprint/.
Back to Citation42. Food Research & Action Center (FRAC). “Hunger is a Health Issue for Older Adults: Food Security, Health, and the Federal Nutrition Programs.” December 2019. https://frac.org/wp-content/uploads/hunger-is-a-health-issue-for-older-adults-1.pdf.
Back to Citation43. The White House Challenge to End Hunger and Build Health Communities (Challenge) was a nationwide call-to-action released on March 24, 2023 to interested parties across all of society to make commitments to advance President Biden's goal to end hunger and reduce diet-related diseases by 2030—all while reducing disparities. More information on the White House Challenge to End Hunger and Build Health Communities can be found: https://www.whitehouse.gov/briefing-room/statements-releases/2023/03/24/fact-sheet-biden-harris-administration-launches-the-white-house-challenge-to-end-hunger-and-build-healthy-communities-announces-new-public-private-sector-actions-to-continue-momentum-from-hist/.
Back to Citation44. Schroeder K, Smaldone A. Food Insecurity: A Concept Analysis. Nurse Forum. 2015 Oct-Dec;50(4):274-84. doi: 10.1111/nuf.12118. Epub 2015 Jan 21. PMID: 25612146; PMCID: PMC4510041.
Back to Citation45. Tsega M, Lewis C, McCarthy D, Shah T, Coutts K. Review of Evidence for Health-Related Social Needs Interventions. July 2019. The Commonwealth Fund. https://www.commwealthfund.org/sites/default/files/2019-07/ROI-evidence-review-final-version.pdf.
Back to Citation46. More information about the HFSS tool can be found at https://www.ers.usda.gov/topics/food- nutrition-assistance/food-security-in-the-u-s/survey-tools /.
Back to Citation47. Hernández D. Understanding `energy insecurity' and why it matters to health. Soc Sci Med. 2016 Oct; 167:1-10. doi: 10.1016/j.socscimed.2016.08.029. Epub 2016 Aug 21. PMID: 27592003; PMCID: PMC5114037.
Back to Citation48. US Energy Information Administration. “One in Three U.S. Households Faced Challenges in Paying Energy Bills in 2015.” 2017 Oct 13. https://www.eia.gov/consumption/residential/reports/2015/energybills/.
Back to Citation49. Hernández D. “Understanding energy insecurity' and why it matters to health.” Soc Sci Med. 2016; 167:1-10.
Back to Citation50. Hernández D. Understanding `energy insecurity' and why it matters to health. Soc Sci Med. 2016 Oct;167:1-10. doi: 10.1016/j.socscimed.2016.08.029. Epub 2016 Aug 21. PMID: 27592003; PMCID: PMC5114037.
Back to Citation51. Hernández D. “What `Merle' Taught Me About Energy Insecurity and Health.” Health Affairs, VOL.37, NO.3: Advancing Health Equity Narrative Matters. March 2018. https://doi.org/10.1377/hlthaff.2017.1413.
Back to Citation52. US Energy Information Administration. “One in Three U.S. Households Faced Challenges in Paying Energy Bills in 2015.” 2017 Oct 13. https://www.eia.gov/consumption/residential/reports/2015/energybills/.
Back to Citation53. Hernández D. Understanding `energy insecurity' and why it matters to health. Soc Sci Med. 2016 Oct;167:1-10. doi: 10.1016/j.socscimed.2016.08.029. Epub 2016 Aug 21. PMID: 27592003; PMCID: PMC5114037.
Back to Citation54. Hernández D, Siegel E. Energy insecurity and its ill health effects: A community perspective on the energy-health nexus in New York City. Energy Res Soc Sci. 2019 Jan;47:78-83. doi: 10.1016/j.erss.2018.08.011. Epub 2018 Sep 8. PMID: 32280598; PMCID: PMC7147484.
Back to Citation55. U.S. Department of Health & Human Services. Office of Community Services. Low Income Home Energy Assistance Program (LIHEAP). https://www.acf.hhs.gov/ocs/programs/liheap.
Back to Citation56. National Council on Aging (NCOA). “How to Make It Easier for Older Adults to Get Energy and Utility Assistance.” Promising Practices Clearinghouse for Professionals. Jan. 13, 2022. https://www.ncoa.org/article/how-to-make-it-easier-for-older-adults-to-get-energy-and-utility-assistance.
Back to Citation57. This validated survey was developed as a clinical indicator of household energy security among pediatric caregivers. Cook, J.T., D.A. Frank., P.H. Casey, R. Rose-Jacobs, M.M. Black, M. Chilton, S. Ettinger de Cuba, et al. “A Brief Indicator of Household Energy Security: Associations with Food Security, Child Health, and Child Development in US Infants and Toddlers.” Pediatrics, vol. 122, no. 4, 2008, pp. e874-e875. https://doi.org/10.1542/peds.2008-0286.
Back to Citation58. In October 2023, we released two new annual Health Equity Confidential Feedback Reports to SNFs: The Discharge to Community (DTC) Health Equity Confidential Feedback Report and the Medicare Spending Per Beneficiary (MSPB) Health Equity Confidential Feedback Report. The PAC Health Equity Confidential Feedback Reports stratified the DTC and MSPB measures by dual-enrollment status and race/ethnicity. For more information on the Health Equity Confidential Feedback Reports, please refer to the Education and Outreach materials available on the SNF QRP Training web page at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/Skilled-Nursing-Facility-Quality-Reporting-Program/SNF-Quality-Reporting-Program-Training.
Back to Citation59. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for Social Risk Factors in Medicare Payment: Identifying Social Risk Factors. Washington, DC: The National Academies Press. https://doi.org/10.17226/21858.
Back to Citation60. National Academies of Sciences, Engineering, and Medicine. 2020. Leading Health Indicators 2030: Advancing Health, Equity, and Well-Being. Washington, DC: The National Academies Press. https://doi.org/10.17226/25682.
Back to Citation62. Billioux, A., Verlander, K., Anthony, S., & Alley, D. (2017). Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool. NAM Perspectives, 7(5). Available at https://doi.org/10.31478/201705b. Accessed on June 9, 2024.
Back to Citation63. Billioux, A., Verlander, K., Anthony, S., & Alley, D. (2017). Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool. NAM Perspectives, 7(5). Available at https://doi.org/10.31478/201705b. Accessed on June 9, 2024.
64. Centers for Medicare & Medicaid Services (2021). Accountable Health Communities Model. Accountable Health Communities Model | CMS Innovation Center. Available at https://innovation.cms.gov/innovation-models/ahcm. Accessed on February 20, 2023.
Back to Citation65. https://www.cms.gov/priorities/innovation/data-and-reports/2023/ahc-second-eval-rpt-fg.
Back to Citation66. https://www.cms.gov/priorities/innovation/data-and-reports/2023/ahc-second-eval-rpt-fg.
Back to Citation67. Accountable Health Communities (AHC) Model Evaluation, Second Evaluation Report. May 2023. This project was funded by the Centers for Medicare & Medicaid Services under contract no. HHSM-500-2014-000371, Task Order75FCMC18F0002. https://www.cms.gov/priorities/innovation/data-and-reports/2023/ahc-second-eval-rpt.
Back to Citation68. In October 2023, we released two new annual Health Equity Confidential Feedback Reports to SNFs: The Discharge to Community (DTC) Health Equity Confidential Feedback Report and the Medicare Spending Per Beneficiary (MSPB) Health Equity Confidential Feedback Report. The PAC Health Equity Confidential Feedback Reports stratified the DTC and MSPB measures by dual-enrollment status and race/ethnicity. For more information on the Health Equity Confidential Feedback Reports, please refer to the Education and Outreach materials available on the SNF QRP Training web page at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/Skilled-Nursing-Facility-Quality-Reporting-Program/SNF-Quality-Reporting-Program-Training.
Back to Citation69. Brooks-LaSure, C. (2021). My First 100 Days and Where We Go from Here: A Strategic Vision for CMS. Centers for Medicare & Medicaid. Available at https://www.cms.gov/blog/my-first-100-days-and-where-we-go-here-strategic-vision-cms.
Back to Citation70. The Biden-Harris Administration's strategic approach to addressing health related social needs can be found in The U.S. Playbook to Address Social Determinants of Health (SDOH) (2023): https://www.whitehouse.gov/wp-content/uploads/2023/11/SDOH-Playbook-3.pdf.
Back to Citation71. World Health Organization. Social determinants of health. Available at https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1.
Back to Citation72. These cue cards are currently available on the SNF QRP Training web page at https://www.cms.gov/medicare/quality/snf-quality-reporting-program/training.
Back to Citation73. Haff, N, Choudhry, N.K., Bhatkhande, G., Li, Y., Antol, D., Renda, A., Laufffenburger, J. Frequency of Quarterly Self-reported Health-Related Social Needs Among Older Adults, 2020. JAMA Network Open. 2022;5(6):e2219645. Doi: 101001/jamanetworkopen.2022.19645. Accessed June 9, 2024.
Back to Citation74. Gundersen C, Engelhard E, Crumbaugh A, Seligman, H.K. Brief assessment of Food insecurity Accurately Identifies High0Risk US Adults. Public Health Nutrition, 2017. Doi: 10.1017/S1368980017000180. https://childrenshealthwatch.org/wp-content/uploads/brief-assessment-of-food-insecurity-accurately-identifies-high-risk-us-adults.pdf. Accessed July 2, 2024.
Back to Citation76. U.S. Department of Health & Human Services. Office of Community Services. Low Income Home Energy Assistance Program (LIHEAP). https://www.acf.hhs.gov/ocs/programs/liheap. Accessed July 2, 2024.
Back to Citation77. Haff, N, Choudhry, N.K., Bhatkhande, G., Li, Y., Antol, D., Renda, A., Laufffenburger, J. Frequency of Quarterly Self-reported Health-Related Social Needs Among Older Adults, 2020. JAMA Network Open. 2022;5(6):e2219645. Doi: 101001/jamanetworkopen.2022.19645. Accessed June 9, 2024.
Back to Citation78. The seven SDOH items are ethnicity, race, preferred language, interpreter services, health literacy, transportation, and social isolation (84 FR 38805 through 38818).
Back to Citation79. Centers for Medicare & Medicaid Services, FY2024 Inpatient Psychiatric Prospective Payment System—Rate Update (88 FR 51107 through 51121).
Back to Citation80. Victoria Transport Policy Institute (2016, August 25). Basic access and basic mobility: Meeting society's most important transportation needs. Retrieved from.
Back to Citation81. Haff, N, Choudhry, N.K., Bhatkhande, G., Li, Y., Antol, D., Renda, A., Laufffenburger, J. Frequency of Quarterly Self-reported Health-Related Social Needs Among Older Adults, 2020. JAMA Network Open. 2022;5(6):e2219645. Doi: 101001/jamanetworkopen.2022.19645. Accessed June 9, 2024.
Back to Citation82. The Post-Acute Care (PAC) and Hospice Quality Reporting Program Cross-Setting TEP summary report will be published in early summer or as soon as technically feasible. SNFs can monitor the Partnership for Quality Measurement website at https://mmshub.cms.gov/get-involved/technical-expert-panel/updates for updates.
Back to Citation83. Centers for Medicare & Medicaid Services. Aligning Quality Measures Across CMS—the Universal Foundation. November 17, 2023. https://www.cms.gov/aligning-quality-measures-across-cms-universal-foundation.
Back to Citation84. A composite measure can summarize multiple measures through the use of one value or piece of information. More information can be found at https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/composite-measures.pdf.
Back to Citation85. CMS Measures Inventory Tool. Adult immunization status measure found at https://cmit.cms.gov/cmit/#/FamilyView?familyId=26.
Back to Citation86. MS Measures Inventory Tool. Clinical Depression Screening and Follow-Up measure found at https://cmit.cms.gov/cmit/#/FamilyView?familyId=672.
Back to Citation87. Centers for Medicare & Medicaid Services. Aligning Quality Measures Across CMS—the Universal Foundation. November 17, 2023. https://www.cms.gov/aligning-quality-measures-across-cms-universal-foundation.
Back to Citation88. Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022. MMWR Recomm Rep 2022;71(No. RR-3):1-95. DOI: https://dx.doi.org/10.15585/mmwr.rr7103a1.
Back to Citation89. Both the PHQ-2 to-9 and Staff Assessment of Resident Mood PHQ-9-OV are collection on the MDS 3.0.
Back to Citation90. Principal Illness Navigation (PIN) services describe services that auxiliary personnel, including care navigators or peer support specialists, may perform incidental to the professional services of a physician or other billing practitioner, under general supervision. Two codes describe PIN services, and two codes describe Principal Illness Navigation-Peer Support (PIN-PS) services, which are intended more for patients with high-risk behavioral health conditions and have slightly different service elements that better describe the scope of practice of peer support specialists. In general, where we describe aspects of PIN, it also applies to PIN-PS unless otherwise specified. MLN9201074 January 2024. https://www.cms.gov/files/document/mln9201074-health-equity-services-2024-physician-fee-schedule-final-rule.pdf-0.
Back to Citation91. Patient Activation Measure® (PAM®). https://www.insigniahealth.com/pam/.
Back to Citation92. FY 2020 SNF PPS final rule (84 FR 38817 through 38818).
Back to Citation93. Due to data availability of SNF SDOH standardized patient assessment data elements, this is based on one quarter of Transportation data.
Back to Citation94. The analysis is limited to residents who responded to the Transportation item at both admission and discharge.
Back to Citation95. Feltner C WI, Berkman N, et al. Screening for Intimate Partner Violence, Elder Abuse, and Abuse of Vulnerable Adults: An Evidence Review for the U.S. Preventive Services Task Force Agency for Healthcare Research and Quality. 2018. Available at https://www.ncbi.nlm.nih.gov/books/NBK533720/.
96. National Academy of Science EaM. A Roadmap to Reducing Child Poverty. The National Academies; 2019.
97. Wise PH. Child poverty and the promise of Human Capacity: childhood as a foundation for healthy aging. Acad Pediatr. 2016;16(suppl 3):S37-S45.
Back to Citation98. Boch S, Keedy H, Chavez L, et al. An integrative review of social determinants of health screenings used in primary care settings. J Health Care Poor Underserved. 2020;31:603-622.
Back to Citation99. Halpin, K, Colvin, JD, Clements, MA, et al. Outcomes of Health-Related Social Needs Screening in a Midwest Pediatric Diabetes Clinic Network. Diabetes. 2023; Vol. 72; Iss: Supplement 1.
100. Nerlinger, AL, Kopsombut, G. Social determinants of health screening in pediatric healthcare settings. Curr Opin Pediatr. 2023 Feb 1;35(1):14-21. Doi: 10.1097/MOP.0000000000001191.
Back to Citation101. Gray, T.W., Podewils, L.J., Rasulo, R.M., Weiss, R.P., Tomcho M.M. Examining the Implementation of Health-Related Social Need (HRSN) Screenings at a Pediatric Community Health Center. Journal of Primary Care & Community Health. 2023. Volume 14: 1-8. https://doi.org/10.1177/21501319231171519.
Back to Citation102. Centers for Medicare and Medicaid Services (CMS) (2023, March 29). Minimum Data Set (MDS) 3.0 for Nursing Homes and Swing Bed Providers. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/nursinghomequalityinits/nhqimds30.
Back to Citation104. https://www.cms.gov/medicare/quality/meaningful-measures-initiative/cms-quality-strategy.
Back to Citation105. https://www.cms.gov/aligning-quality-measures-across-cms-universal-foundation.
Back to Citation108. 89 FR 40876 (May 10, 2024); https://www.federalregister.gov/documents/2024/05/10/2024-08273/medicare-and-medicaid-programs-minimum-staffing-standards-for-long-term-care-facilities-and-medicaid.
Back to Citation109. 89 FR 40876 (May 10, 2024); https://www.federalregister.gov/documents/2024/05/10/2024-08273/medicare-and-medicaid-programs-minimum-staffing-standards-for-long-term-care-facilities-and-medicaid.
Back to Citation111. U.S. Bureau of Labor Statistics' (BLS) May 2022 National Occupational Employment and Wage Estimates. https://www.bls.gov/oes/current/oes_nat.htm.
Back to Citation112. U.S. Bureau of Labor Statistics' (BLS) May 2022 National Occupational Employment and Wage Estimates. https://www.bls.gov/oes/current/oes_nat.htm.
Back to Citation113. Medicare Benefit Policy Manual 100-02; Chapter 8—Coverage of Extended Care (SNF) Services Under Hospital Insurance; Section 30.2—Skilled Nursing and Skilled Rehabilitation Services.
Back to CitationBILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
[FR Doc. 2024-16907 Filed 7-31-24; 4:15 pm]
BILLING CODE 4120-01-P
Document Information
- Effective Date:
- 10/1/2024
- Published:
- 08/06/2024
- Department:
- Centers for Medicare & Medicaid Services
- Entry Type:
- Rule
- Action:
- Final rule.
- Document Number:
- 2024-16907
- Dates:
- These regulations are effective on October 1, 2024.
- Pages:
- 64048-64163 (116 pages)
- Docket Numbers:
- CMS-1802-F
- RINs:
- 0938-AV30: FY 2025 Skilled Nursing Facility (SNFs) Prospective Payment System and Consolidated Billing and Updates to the Value-Based Purchasing and Quality Reporting Programs (CMS-1802)
- RIN Links:
- https://www.federalregister.gov/regulations/0938-AV30/fy-2025-skilled-nursing-facility-snfs-prospective-payment-system-and-consolidated-billing-and-update
- Topics:
- Administrative practice and procedure, Diseases, Health facilities, Health professions, Medicare, Puerto Rico, Reporting and recordkeeping requirements
- PDF File:
- 2024-16907.pdf
- CFR: (2)
- 42 CFR 413
- 42 CFR 488