05-240. Endangered and Threatened Wildlife and Plants; Mariana Fruit Bat (Pteropus mariannus mariannus
-
Start Preamble
AGENCY:
Fish and Wildlife Service, Interior.
ACTION:
Final rule.
SUMMARY:
We, the U.S. Fish and Wildlife Service (Service), reclassify from endangered to threatened status the Mariana fruit bat (Pteropus mariannus mariannus) from Guam, Start Printed Page 1191under the authority of the Endangered Species Act of 1973, as amended (Act), and determine the Mariana fruit bat from the Commonwealth of the Northern Mariana Islands (CNMI) to be a threatened species under the authority of the Act. This rule lists the Mariana fruit bat as threatened throughout its range.
The Mariana fruit bat was listed previously as endangered on Guam. The bat populations on the southern islands of the CNMI (Aguiguan, Tinian, and Saipan) were candidates for listing. The best available scientific information indicates that Mariana fruit bats on Guam and throughout the CNMI comprise one subspecies. The protections of the Act, therefore, apply to this subspecies throughout its known range in the Mariana archipelago.
DATES:
This final rule is effective February 7, 2005.
ADDRESSES:
Comments and materials received, as well as supporting documentation used in the preparation of this final rule, will be available for public inspection, by appointment, during normal business hours at the Pacific Islands Fish and Wildlife Office, U.S. Fish and Wildlife Service, 300 Ala Moana Boulevard, Room 3-122, Box 50088, Honolulu, HI 96850.
Start Further InfoFOR FURTHER INFORMATION CONTACT:
Gina Shultz, Assistant Field Supervisor, Pacific Islands Fish and Wildlife Office (see ADDRESSES section) (telephone 808/792-9400; facsimile 808/792-9581).
End Further Info End Preamble Start Supplemental InformationSUPPLEMENTARY INFORMATION:
Background
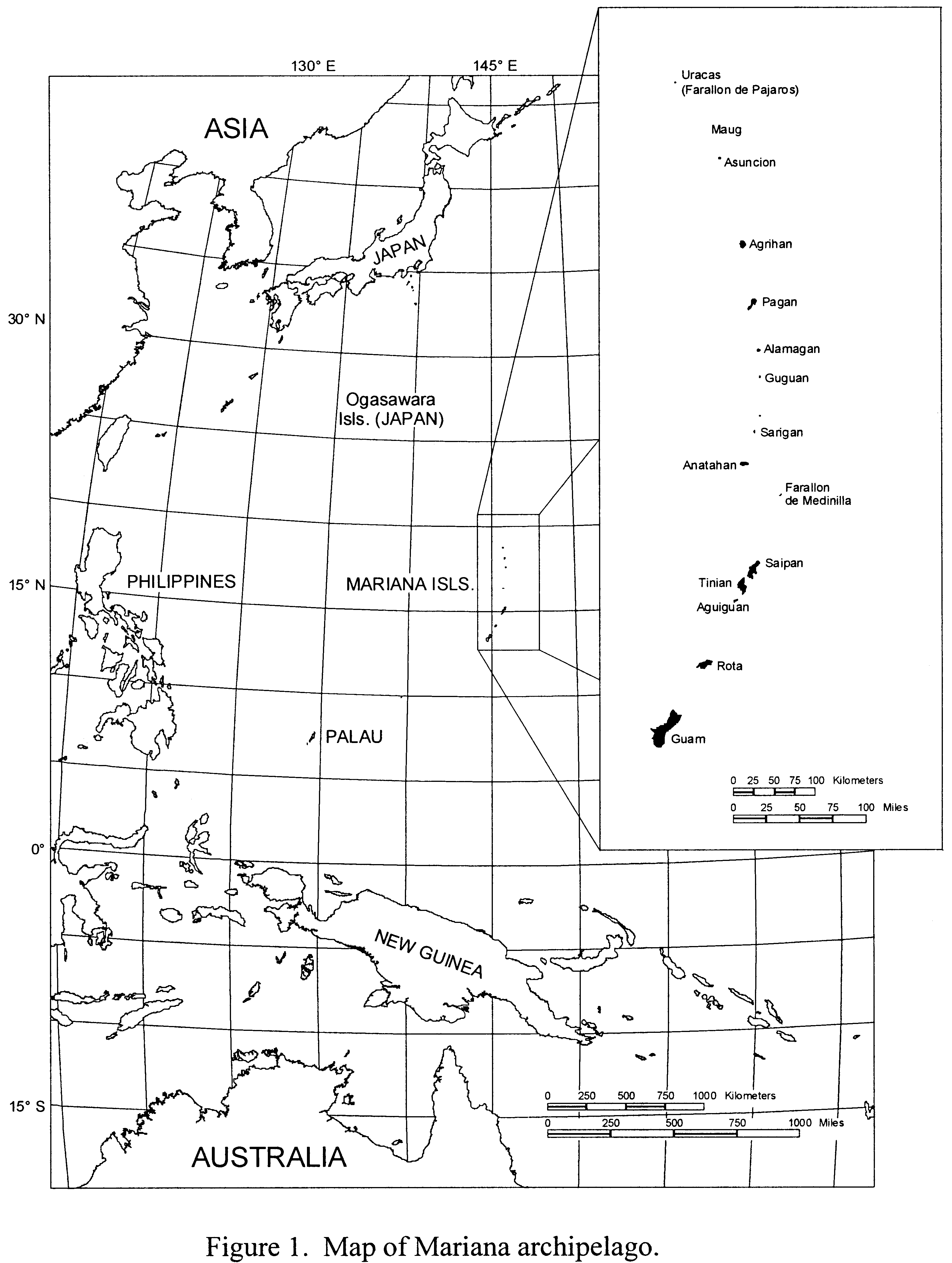
The Mariana archipelago consists of the 15-island Commonwealth of the Northern Mariana Islands (CNMI) and the Territory of Guam, both within the jurisdiction of the United States. This archipelago extends 470 miles (mi) (750 kilometers (km)) from 13°14′ N, 144°45′ W to 20°3′ N, 144°54′ W and is approximately 900 mi (1,500 km) east of the Philippine Islands (Figure 1). Nine of the 10 northern islands (Anatahan, Sarigan, Guguan, Alamagan, Pagan, Agrihan, Asuncion, Maug, and Uracas) are volcanic in origin, and Farallon de Medinilla and the five southern islands (Guam, Rota, Aguiguan, Tinian, and Saipan) are uplifted limestone plateaus with volcanic outcrops. Mariana fruit bats have historically inhabited all of these islands except Uracas, the northernmost island (Wiles and Glass 1990). Of the largest southern islands (Guam, Rota, Tinian, and Saipan), Guam supports the majority of the human population. The northern islands (north of Saipan) are either unoccupied or support only a few families. The climate is tropical, with daily mean temperatures of 75 to 90° Fahrenheit (24 to 32° Celsius), high humidity, and average annual rainfall of 80 to 100 inches (in) (200 to 260 centimeters (cm)). Typhoons may strike the Mariana Islands during any month of the year, but are most frequent between July and October.
Start Printed Page 1192 Start Printed Page 1193Species Description and Biology
The Mariana fruit bat is a medium-sized fruit bat in the family Pteropididae that weighs 0.66 to 1.15 pounds (330 to 577 grams) and has a forearm length ranging from 5.3 to 6.1 in (13.4 to 15.6 cm); males are slightly larger than females. The underside (abdomen) is colored black to brown, with gray hair interspersed, creating a grizzled appearance. The shoulders (mantle) and sides of the neck are usually bright golden brown, but may be paler in some individuals. The head varies from brown to dark brown. The well-formed and rounded ears and large eyes give the face a canine appearance; members of the family Pteropodidae often are referred to as flying foxes.
The Mariana fruit bat is highly colonial, forming colonies of a few to over 800 animals (Wiles 1987a; Pierson and Rainey 1992; Worthington and Taisacan 1995). Bats group themselves into harems (1 male and 2 to 15 females) or bachelor groups (predominantly males), or reside as single males on the edge of the colony (Wiles 1987a). On Guam, the average estimated sex ratio in a single colony varied from 37.5 to 72.7 males per 100 females (Wiles 1982).
Reproduction is believed to occur throughout the year in Pteropus mariannus yapensis on Yap (Falanruw 1988). Mating and the presence of nursing Pteropus mariannus mariannus young have been observed year-round on Guam (Perez 1972; Wiles 1983) with no apparent peak in births (Wiles 1987a). Glass and Taisacan (1988) suggested a similar pattern on Rota, but also indicated that a peak birthing season may occur during May and June, as has been observed in other fruit bats (Pierson and Rainey 1992). Female bats of the family Pteropodidae have one offspring per year (Pierson and Rainey 1992), pups may be born in any month of the year. Observations on Guam between July 1982 and May 1985 found 262 female bats, each with a single young (Service 1990). This reproductive rate, very low for a mammal of this size, results in a low maximum population growth rate, and thus a slow rate of recovery when a population is diminished (Pierson and Rainey 1992). Length of gestation and age of sexual maturity are unknown for the Mariana fruit bat; other related bats have a gestation period of approximately 4.6 to 6.3 months (Pierson and Rainey 1992). Age of sexual maturity is not known for the Mariana fruit bat, but Pteropus species typically do not breed before 18 months of age (Pierson and Rainey 1992).
Taxonomy and Interisland Movements
The fruit bats of the Mariana Islands consistently have been treated as one or more endemic subspecies or species; that is, they occur nowhere outside the archipelago (Andersen 1912; Kuroda 1938; Corbet and Hill 1980, 1986, 1991; Koopman 1982, 1993; Flannery 1995). Following the taxonomic treatments of Kuroda (1938) and Koopman (1993), which are known to be based on examination of numerous specimens, and the most recent treatment by Flannery (1995), Pteropus mariannus is a widely dispersed species occurring north of the equator in portions of Micronesia north to the Japanese Ryukyu Islands. Various authors have attributed different numbers of subspecies to P. mariannus. Kuroda (1938) and Koopman (1982, 1993) recognize seven subspecies; Flannery recognizes three.
Pteropus fruit bats are well known to be strong fliers and traverse long distances (Eby 1991; Palmer and Woinarski 1999; Nelson 2003). Evidence that Mariana fruit bats fly between islands in the archipelago supports consideration of these bats as a single subspecies made up of numerous island populations in the Marianas (Lemke 1986; Service 1990; Wiles and Glass 1990; Worthington and Taisacan 1996). The geography of the archipelago, as well as the flight capability of fruit bats, facilitates interisland exchange. Distances between islands in the Mariana archipelago range from 3 to 62 mi (5 to 100 km). Each island in the chain is visible from neighboring islands (Wiles and Glass 1990).
The August 27, 1984, Federal listing (49 FR 33881) of fruit bats resident on Guam was based on an assumption that these bats were a distinct subspecies isolated from other bat populations in the CNMI. However, current evidence exists that large numbers of bats from Rota have visited Guam for periods of months. Temporary spikes in the Guam fruit bat population were observed in 1992-1993 (from about 350 to 550 bats) and in 1998 (from about 150 to 760 bats) (Anne Brooke, Service, in litt. 2003). These temporary increases lasted for several months. More modest but equally sudden increases in the Guam population were noted 2 and 4 days following Typhoons Chataan and Pongsona, respectively, in 2002 (Dustin Janecke, University of Guam, in litt. 2003). The most likely explanation is a temporary relocation of bats from Rota, which lies 48 mi (77 km) from Guam, is visible from Guam's north shore, and harbors one of the largest fruit bat populations in the archipelago. For example, the 2002 spike on Guam after Typhoon Pongsona was concurrent with an observed dip in fruit bat numbers on Rota (Jake Esselstyn, University of Kansas (formerly CNMI Department of Fish and Wildlife (DFW)), pers. comm. 2004b). Several other instances of apparent immigrations from Rota to Guam documented in the late 1970s and 1980s are described in detail by Wiles and Glass (1990). Although we cannot be certain that “visiting” bats interbreed with resident Guam bats during their months on the island, the fact that Mariana fruit bats breed throughout the year (Wiles 1983, 1987a) leaves this possibility open. The presence of fruit bats on the islands of Tinian and Aguiguan, which are close to one another and to Saipan, is ephemeral (Worthington and Taisacan 1996), indicating that interisland travel likely occurs among these three islands as well.
An example of likely interisland movement in the northern islands of the CNMI comes from Sarigan. Fruit bat surveys on Sarigan documented a roughly stable level of approximately 125-235 bats between 1983 and 2000 (Wiles et al. 1989; Fancy et al. 1999; Wiles and Johnson 2004). In 2001, surveys estimated 300-400 bats (Wiles and Johnson 2004). Recruitment of juvenile bats alone cannot account for this increase, and Wiles and Johnson (2004) posit Anatahan, 23 mi (37 km) to the south, as the likely source for immigrants. Wiles et al. (1989) twice observed individual fruit bats 0.8 mi (2 km) from Guguan, flying south in the direction of Sarigan, which lies 39 mi (63 km) away. Anecdotal observations of likely transits among other northern islands are described in Wiles and Glass (1990) and by other species experts (Worthington and Taisacan 1996; Wiles and Johnson 2004).
Like fruit bats, many other highly mobile vertebrates of Pacific Islands, especially birds, are treated as a single species or subspecies inhabiting multiple islands in an archipelago (Mayr 1945; Pratt et al. 1987; Watling 2001). Immigration rates of perhaps one individual per generation could be necessary for an island population to maintain genetic homogeneity with the populations on other islands (Mills and Allendorf 1996; Wang 2004; Gary McCracken, University of Tennessee, pers. comm. 2004). The chances of witnessing such a low rate of immigration are slight. The evidence described above for interisland movement suggests even greater rates of movement and probable gene flow among the fruit bat populations on various islands in the Mariana Start Printed Page 1194archipelago than the minimum needed to maintain genetic homogeneity.
Preliminary results of a recent study of genetic variation in a similarly gregarious (Pierson and Rainey 1992) and mobile species of fruit bat elsewhere in the Pacific provide further, if circumstantial, support for the existence of a single subspecies of fruit bats in the Marianas. Genetic material collected from the white-collared fruit bat (Pteropus tonganus) in Samoa and Fiji shows a lack of genetic isolation within island groups (Utzurrum et al. 2000; G. McCracken, pers. comm. 2004). Little anecdotal observation of interisland movements exists for P. tonganus, yet apparently it experiences immigration at sufficient intervals to prevent genetic isolation.
Currently, there are two recognized subspecies restricted to the Mariana Islands: the Mariana fruit bat (Pteropus mariannus mariannus) and the Pagan fruit bat (Pteropus mariannus paganensis). Other subspecies are endemic to other archipelagos and do not occur in the Marianas. The taxonomic status of the Pagan fruit bat is questionable. Yamashina (1932) collected three male fruit bats and one female from the islands of Pagan and Alamagan in 1931, and stated: “[t]his species, as compared to the Pteropus mariannus mariannus that inhabit Guam, is distinctly darker in coloration, having brownish wings.” He made no further comparisons, and thus the distinction of this taxon is based on a single, equivocal interpretation of the coloration of four specimens. Although future studies may confirm the existence of a distinct taxon of fruit bats in the northern islands, at this time, based on the best available science including peer reviewer comments, we do not consider Pteropus mariannus paganensis as distinct from Pteropus mariannus mariannus to represent a single taxon.
Habitat
Mariana fruit bats forage and roost primarily in native forest and forage occasionally in coconut (Cocos nucifera) groves and strand vegetation (Wiles 1987b; Worthington and Taisacan 1996). Wiles (1987b) described six bat roost sites on Guam, all within native limestone forest. Major roost trees included Ficus spp. and Neisosperma oppositifolia. On Rota, fruit bats used primary and secondary limestone forest for roosting and foraging (Glass and Taisacan 1988). At least nine tree species were used for roosting, including Elaeocarpus sphaericus, Macaranga thompsonii, Guamia mariannae, Hernandia spp., Artocarpus mariannensis, Ficus prolixia, Barringtonia asiatica, Randia cochinchinensis, and the introduced Theobroma cacao (Glass and Taisacan 1988). A small bat colony also was observed roosting in Casuarina equisetifolia on Aguiguan (Worthington and Taisacan 1996). At least 22 plant species are used as food sources by the Mariana fruit bat. Food items include the fruits of 17 species of plants, especially the native Artocarpus mariannensis, Cycas circinalis, Ficus spp., Pandanus tectorius, Terminalia catappa, and the introduced Artocarpus altilis and Carica papaya; the flowers of seven plants, including the native Ceiba pentandra and Erythrina variegata, and the introduced Cocos nucifera; and leaf stems and twig tips of Artocarpus spp. (Wiles 1987a; Service 1990). Although Mariana fruit bats have been observed to feed on and roost in cultivated, introduced food plants, nonnative species make up only a small fraction of the plants they use (Wiles 1987b; Worthington and Taisacan 1996). Fruit bats are important components of tropical forest ecosystems because they disperse plant seeds and thereby help maintain forest diversity and contribute to plant regeneration following typhoons and other catastrophic events (Cox et al. 1992).
CNMI Southern Islands
The relatively large size and moderate topography of the southern islands led to their being, along with Guam, the most heavily populated and intensively cultivated islands in the archipelago. All of the southern Marianas are hypothesized to have been densely forested when first settled by humans some 3,500 years ago (Mueller-Dombois and Fosberg 1998). The loss and alteration of native habitats on these islands began with prehistoric cultivation, accelerated with the 17th century introduction of livestock and mechanized agriculture by Europeans, and likely peaked during the mid-20th century with landscape-scale habitat conversion by commercial agriculture, military infrastructure, and bombardment (Bowers 1950; Fosberg 1960; Stone 1970). This long continuous and intense human disturbance is reflected by the near absence of Mariana fruit bats from Saipan, Tinian, and Guam.
On Saipan and Tinian, agriculture and free-roaming livestock had converted much of the islands' forest to fields and pastures as early as the 18th century (Barrat 1988 in Stinson et al. 1992). Human populations on these islands increased steadily, and virtually all arable land was used to grow cash crops or food (Bowers 1950). Sugar plantations dominated the landscapes of Saipan, Tinian, and Aguiguan prior to World War II (Fosberg 1960). Saipan and Tinian were invaded during World War II, and during and after the war, bombing and extensive military development resulted in the loss of additional fruit bat habitat (Bowers 1950; Fosberg 1960). After the war, Saipan and Tinian were estimated to retain 5 and 2 percent native forest cover, respectively (Bowers 1950), and these proportions apparently were not significantly different in 1982 (Engbring et al. 1986). The introduction of nonnative species such as tangantangan for erosion control has left these islands dominated by alien vegetation that inhibits the growth of native forest (Fosberg 1960; Craig 1993). Feral ungulates are present on both islands, resulting in further degradation and fragmentation. Finally, Saipan is the most heavily populated and industrialized island in the CNMI (CNMI Statistical Yearbook 2001). Aguiguan was not invaded during the war, and has retained a greater proportion of its native forest (20 percent; Bowers 1950).
Similar to Saipan and Tinian, large areas of Rota were converted to sugar plantations in the early part of the 20th century (Fosberg 1960). Rota has more rugged topography, however, and was not invaded during World War II. These two factors are thought to explain the greater amount of native forest cover (25 percent) remaining on Rota following the war (Baker 1946; Bowers 1950). Engbring et al. (1986) estimated that roughly 60 percent of Rota's land area supported native vegetation in 1982. It is not clear whether Engbring's estimate represents some level of native forest recovery since Bowers' (1950) post-war estimate, or is a different interpretation and measurement of forest cover.
Most of Guam's native vegetation has been replaced by land development and invasive species. Guam is the population and commercial center of the archipelago, and commercial and residential development are ongoing. Like the other southern islands, parts of Guam were seeded with tangantangan following World War II to control erosion (Fosberg 1960). Large areas of southern Guam are dominated by savannas; these landscapes are thought to have originated as a result of aboriginal burning (Fosberg 1960). In 1981, northern Guam, which supports the last extensive native forest remaining on the island, was thought to retain no more than 37 percent native forest cover (Engbring and Ramsey 1984). Feral ungulates are abundant and Start Printed Page 1195widespread throughout the island and cause significant damage to all remaining native forest (Fosberg 1960; Stone 1970; A. Brooke, pers. comm. 2004). Lands owned by the U.S. Air Force (Air Force) at Andersen Air Force Base in northern Guam include the largest contiguous forested areas left in northern Guam; the Air Force permits hunting of feral ungulates on parts of the base (U.S. Air Force 2001).
CNMI Northern Islands
Compared with the history of habitat loss in the southern islands, degradation or loss of native forest in the northern islands of the CNMI is a recent phenomenon; therefore, these islands have retained more habitat to support Mariana fruit bats. Some of the northern islands have supported small human settlements, and most of these have been occupied only sporadically. Feral ungulates have been present in the northern islands only since the mid-20th century. For example, Anatahan has had feral goats and pigs for roughly 40 years (Kessler 1997), and forest degradation and erosion were observed to escalate sharply during the 1990s (Marshall et al. 1995; Kessler 2000a; Worthington et al. 2001), possibly because feral ungulate damage was exacerbated by El Nino-related drought in the late 1990s (Kessler 2000a).
Although changes in forest cover were not quantified, evidence from point photo monitoring and other land-based photography conducted on Anatahan in 1983, 1996, and 2000 documented widespread loss of forest, reduced canopy cover in remaining forest, and increased erosion resulting from feral ungulate damage (Marshall et al. 1995; Kessler 1997, 2000a; Worthington et al. 2001). An ungulate eradication project was begun in 2002, but was not completed when Anatahan volcano erupted in 2003. This eruption further compromised the island's forest habitat, and continuing volcanic activity has hindered completion of the ungulate eradication project. A large population of feral pigs still occurs on the island and some goats remain; aerial hunting for goats is ongoing (Curt Kessler, Service, pers. comm. 2004b). Some vegetation recovery has been observed as a result of goat control, but an invasive alien vine, Mikania micrantha, has spread rapidly and may inhibit the growth of native vegetation (C. Kessler, pers. comm. 2004b). This plant is known to smother and displace native vegetation on other Pacific islands (U.S. Department of Agriculture (USDA) 2004).
On Pagan, livestock was maintained in captivity by island residents until the volcanic eruption in 1981, when the human population was evacuated. In the subsequent 23 years, large populations of feral goats, pigs, and cattle have become established on the island and have caused significant damage (Rice and Stinson 1992; Kessler 1997). The degradation and loss of native forest on Pagan is thought to be occurring more rapidly on there than on Anatahan because of the added impact of cattle, which are absent from Anatahan (Kessler 1997). The reductions in fruit bat numbers on Pagan are attributed to feral ungulates causing major damage to the native forest and preventing its regeneration following the 1981 eruption, large areas especially in the northern part of the island being converted to grassland or devegetated and eroded (Kessler 1997), and the spread of the invasive tree Casuarina equisetifolia in monotypic stands (Rice and Stinson 1992; Cruz et al. 2000e). In 1992, Casuarina coverage in the upland areas of the island was estimated at roughly 60 percent (Rice and Stinson 1992). Although this tree is used for roosting by Mariana fruit bats (C. Kessler, pers. comm. 2004b), it does not provide food resources, and it likely displaces native forest, as it has done elsewhere in the Pacific (Cruz et al. 2000e; USDA 2004).
Vegetation surveys in 2000 on Agrihan, the third-largest of the northern islands, documented damage from feral ungulates in the 30 to 40 percent of the island that supports forest habitat (Cruz et al. 2000f). The extremely steep and dissected topography of Agrihan is thought to restrict the distribution of feral ungulates as well as access by humans, and keep goats and pigs geographically separated (Rice et al. 1990; Rice and Stinson 1992), thereby protecting roost sites and sufficient forest habitat to support foraging fruit bats.
Feral goats, pigs, and cattle are present on Alamagan and the extent of native forest remaining on the island is limited to ravines on the south and west slopes and a small plateau in the center of the island (Wiles et al. 1989). Rice (1992) described Alamagan as having “one of the worst feral ungulate problems in the CNMI,” and during vegetation surveys in 2000, Cruz et al. (2000b) found the remaining forests to be in decline.
Maug, Asuncion, Guguan, and (since 1998) Sarigan are free of feral ungulates, but the small size of these islands and the limited extent of their forest habitat ultimately limits the number of fruit bats they can support. Maug is only 10 to 14 percent forested (Wiles et al. 1989), and thus supports little habitat for fruit bats. Forest on Asuncion and Guguan is limited to the lower western and southern areas; the northern and steep upper parts of these islands are bare volcanic ash or grassland (Wiles et al. 1989). Roughly 32 percent or 400 acres (ac) (162 hectares (ha)) of Sarigan is forested, but most of this is monotypic coconut forest that provides only minimal forage for fruit bats; only about 72 ac (29 ha) supports relatively diverse native forest that provides both roosting and foraging resources for fruit bats (Wiles and Johnson 2004). Although the eradication of ungulates from Sarigan and initial vegetation recovery may play a role in increased numbers of fruit bats on the island, invasive, alien plants such as tangantangan (Leucaena leucocephala) and Operculina ventricosa also are present on the island and may impede the recovery of native forest over the long term (Kessler 2000b). These plants are known to degrade native vegetation in the Mariana Islands and elsewhere in the Pacific (USDA 2004).
Landownership of Fruit Bat Habitat in the Mariana Islands
Most of the known fruit bat roost sites in the Mariana Islands are located on public lands. On Guam, the single remaining roost and most fruit bat foraging habitat is found on U.S. military lands; some foraging habitat occurs on private lands and lands belonging to the Government of Guam (Wiles 1998). The Air Force controls access to Andersen Air Force Base in northern Guam, and the high security and frequent patrols practiced on base effectively create a refugium for fruit bats (Morton 1996). The remote and relatively pristine area where the roost is located was set aside by the military in 1973 as a research natural area; access to and activities in this area are tightly restricted, but no brown treesnake control currently takes place specifically at the roost site (Air Force 2001). Service and Government of Guam wildlife biologists and authorized researchers are permitted access to the area and to the colony to monitor and conduct research on fruit bats. Similarly, the U.S. Navy (Navy) and the Service restrict access to their lands, which include native forest that provides foraging habitat for the fruit bat.
The remaining roost site is managed as part of the Guam National Wildlife Refuge (Refuge) overlay under a cooperative agreement with the Air Force. The Refuge was created on October 1, 1993, with additional lands (overlay portion) incorporated in 1994 by cooperative agreements between the Start Printed Page 1196Service, the Air Force and the Navy. The establishment and management of the overlay portion of the Refuge on Navy and Air Force lands provides a commitment by the three agencies to develop coordinated programs centered on the protection of endangered and threatened species and other native flora and fauna. Active implementation of such programs by these agencies contributes to the continued survival of the Mariana fruit bat on Guam, as important foraging and roosting habitat is located within the Refuge boundaries. However, the lack of brown treesnake control in the immediate area where the fruit bats roost is a serious deficiency in existing programs to protect endangered species on the overlay refuge.
There is no U.S. Government-owned land in the CNMI, but the Navy leases Farallon de Medinilla and part of Tinian. All other public lands are administered by the CNMI government. Saipan has little public land that is not leased and developed, but a few areas still support native forest that is occasionally used by fruit bats. Tinian has large tracts of public land that contain small stands of native forest suitable for bats, and a large portion of public land on the northern end of the island is under lease to the Navy for military activities (Lusk et al. 1997). All of Aguiguan is owned by the CNMI government. Approximately 60 percent of the land on Rota is publicly owned, although much of this has been leased to private individuals. The primary roosting areas on Rota are on Commonwealth lands, but some private lands still retain native limestone forest that may support fruit bats. The northern islands are mostly public lands, with some land developed as small homestead lots.
Population Surveys and Status
Obtaining accurate estimates of fruit bat populations in Pacific archipelagos depends on regular monitoring, standardized survey methods, and consideration of the unique ecology and physiographic environment of bat populations in various island groups (Utzurrum et al. 2004). The difficult terrain of the Mariana Islands, remote location of the northern islands of the CNMI, and the high costs associated with transits of the island group by sea and aerial surveys of individual islands have hindered the establishment of a standard monitoring program for the archipelago.
No known historical records exist to document the status of the Mariana fruit bat prior to the 20th century. The history of fruit bat surveys and changes in numbers summarized below represent a variety of methods and analyses. Archipelago-wide surveys were conducted in 1983 (Wiles et al. 1989) and 2001 (Johnson 2001).
The relatively isolated northern islands support the majority of the fruit bats in the archipelago, but because of their remote location, these islands have not been surveyed as frequently as the southern islands. Individual surveys have been conducted on several of the southern islands at relatively frequent intervals, and comprehensive surveys of the northern islands were conducted in 1983, 2000, and 2001 (Wiles et al. 1989; Cruz et al. 2000a-f; Johnson 2001). Opportunistic surveys have also occurred sporadically throughout the archipelago. The methods used in the northern islands in 2001 were significantly different from those used in 1983 and 2000; we therefore consider only Wiles et al. (1989) and Cruz et al. (2000a-f) for purposes of comparison (Table 1). A conservative interpretation of this comparison indicates a decline between 1983 and 2000, especially on the two islands that supported the largest numbers of fruit bats in the archipelago 20 years ago (Table 1).
Two of the northern islands are not included in this table: Uracas, the most northerly, where fruit bats are not known to occur; and Farallon de Medinilla, where fruit bats have been observed on only one occasion. See text and Table 2 for information about additional and more recent surveys and observations of fruit bats on the southern islands of the CNMI and Guam, and on Farallon de Medinilla, Anatahan, Sarigan, and Pagan.
Start Printed Page 1197Table 1.—Summary of Mariana Fruit Bat Survey Results: Minimum Estimates
Island Area Sq. mi (Sq. km) 1983 1 2000 2 Maug 0.8 (2.0) <25 (3) Asuncion 2.9 (7.4) 400 (3) Agrihan 18.3 (47.4) 1,000 1,000 Pagan 18.4 (47.7) 2,500 1,500 Alamagan 4.3 (11.0) 0 200 Guguan 1.5 (4.0) 400 350 Sarigan 1.9 (5.0) 125 200 Anatahan 12.5 (32.3) 3,000 1,000 Total (Northern Islands) 7,450 [Total six islands] [7,025] 4,250 Saipan 47.5 (122.9) <50 (3) Tinian 39.3 (101.8) <25 (3) Aguiguan 2.7 (7.0) <10 150-200 Rota 37.0 (95.7) 800-1,000 (3) Guam 212.0 (549.0) 425-500 (3) Total (All Islands) 8,760-9,035 N/A 1 Wiles et al. 1989. Dates: August 17-September 10, 1983; 1-4 days/island. Count methods: Evening dispersal counts at colonies; evening station counts of solitary fruit bats. 2 Cruz et al. 2000a-f. Dates: June 4-August 16, 2000; 7-9 days/island. Count methods: Evening dispersal counts at colonies, evening and morning station counts of solitary fruit bats. 3 Not surveyed. Status of CNMI Southern Islands
Fruit bats on the southern islands of the CNMI, Tinian, Saipan, Aguiguan, and Rota were not surveyed prior to the 1970s, but historical accounts indicate that fruit bats once were much more common on these islands than they are now. Schnee (1911) reported that bats were commonly seen and heard on Saipan, where they were heavily hunted by local residents. The Navy restricted civilian access to the northern part of Saipan until the early 1970s, effectively providing fruit bats with protected roost sites. The fruit bat population on Saipan was observed to decline rapidly after the Navy turned over the control to the CNMI government and access to the whole island became unrestricted (Wiles et al. 1989). Observations during the 1980s and 1990s suggested that the Saipan population was small; typically fewer than 50 bats were observed (Lemke 1984; Wiles et al. 1989; Wiles 1996; Worthington and Taisacan 1996). Surveys on Saipan in 2001 estimated that roughly 50 bats were present (Johnson 2001).
Fritz (1901) reported a large number of bats on Tinian in 1900 and Fritz (1904) reported that bats were common on all the southern islands. Fruit bats are only occasionally seen on Tinian today (Marshall et al. 1995; Krueger and O'Daniel 1999; Johnson 2001). Observations during the 1990s suggested that the presence of bats on Tinian was intermittent and their numbers were low (Lemke 1984; Wiles 1996; Worthington and Taisacan 1996). Surveys on Tinian conducted in 2001 found no fruit bats (Johnson 2001). In 1995, between 100 and 125 bats were believed present on Aguiguan (Wiles 1996). During a 10-day visit in 2003, however, no fruit bat colonies were observed on Aguiguan despite extensive coverage, and only a few individual fruit bats were seen (J. Esselstyn, pers. comm. 2004a).
The fruit bats on Rota have been surveyed on a regular basis by a large number of workers since 1986, using methods described by Stinson et al. (1992): primarily evening dispersal counts (EDCs), with some station counts of solitary or extracolonial bats and direct counts of colonial roosts (Glass and Taisacan 1988; Stinson et al. 1992; Worthington and Taisacan 1995, 1996; Johnson 2001; J. Esselstyn in litt. 2003, pers. comm. 2004a). This monitoring effort has yielded numbers that vary widely both intra- and interannually (e.g., Glass and Taisacan 1988; Worthington and Taisacan 1995, 1996). Analysis of the census data on Rota is underway (Laura Williams, CNMI DFW, pers. comm. 2004).
Fruit bat numbers declined following Typhoon Roy in 1988 from an estimated 2,400 animals to just under 1,000 (Worthington and Taisacan 1996). Prior to Typhoon Pongsona in 2002, however, the Rota bat population had risen back to approximately 2,500 (J. Esselstyn, in litt. 2003). In the months following the storm, repeated surveys indicated that numbers had again declined sharply to about 600 (J. Esselstyn, pers. comm. 2004b). Continued surveys of Rota's fruit bats indicate that the population was once again rising in 2004; in April it was estimated at roughly 1,500 animals (J. Esselstyn, pers. comm. 2004a, 2004b). The Rota population fluctuates and may be resilient, but severe storms at short intervals could erode this resilience. The most recent available estimate of fruit bat numbers on Rota is 1,100 (C. Kessler, pers. comm. 2004b). This estimate was made in May 2004, prior to Typhoon Chaba. The bats from Rota are believed to move among the southern islands, and this population thus is considered to be important to the long-term stability of fruit bats in the southern islands of the Mariana archipelago (Wiles and Glass 1990), and to the existence of the colony on Guam (Catherine Leberer, Guam Division of Aquatic and Wildlife Resources (DAWR), in litt. 2004).
Status of CNMI Northern Islands
The 1983 survey of the northern islands resulted in an estimate of 7,450 bats for Anatahan, Sarigan, Guguan, Alamagan, Pagan, Agrihan, Asuncion, and Maug (Wiles et al. 1989, Tables 1 and 2). Because field observation of Mariana fruit bats indicate that this species is gregarious and typically roosts in large colonies during the day, this and subsequent surveys focused on locating colonies. Wiles et al. (1989) located colonies by circumnavigating islands by boat, traversing portions of each island on foot, and interviewing residents on islands with human inhabitants. EDCs were conducted at each colony beginning at 1 to 3 hours before nightfall and continuing until complete darkness. These surveys were carried out by observers placed so that fruit bats departing the colony were silhouetted against the sky or the ocean. Rates of fruit bat departure from colonies were observed to be greatest between 10 and 40 minutes after sunset, but because departures continued after darkness when they are difficult to see, EDCs represent minimum counts (Wiles et al. 1989). In addition, evening counts of solitary or extra-colonial bats were made from vantage points determined to overlap least with the apparent dispersal trajectory of colony bats. Islandwide estimates were based on the number of fruit bats recorded, island size, extent of forest cover and abundance and diversity of food-plant species (Wiles et al. 1989).
Surveys of the northern islands undertaken in 2000 (Cruz et al. 2000a-f) employed a combination of the same methods used by Wiles et al. (1989) in 1983 and, on Anatahan, by Worthington et al. (2001) in 1995: land- and sea-based colony searches, EDCs, station-counts of extra-colonial bats, and direct day-time counts at roosts. On each island they visited, Cruz et al. (2000a-f) spent periods conducting fruit bat surveys equal to or greater than periods spent by Wiles et al. (1989) on the same six islands. The individual island-wide estimates of Cruz et al. (2000a-f) thus are comparable to those of Wiles et al. (1989), but owing to logistical and fiscal constraints, Cruz et al. (2000a-f) did not visit Asuncion and Maug. The 2000 surveys yielded an estimate of 4,450 fruit bats for the 6 northern islands they visited (Cruz et al. 2000a-f). The 1983 surveys yielded an estimate of 7,025 fruit bats for the same six islands (Wiles et al. 1989). A conservative interpretation of these data indicates a 37 percent decline in fruit bat numbers between 1983 and 2000 among these six northern islands.
The majority of this decline was recorded on two of the three largest northern islands, Anatahan (12.5 square mi (32.3 square km)) and Pagan (18.4 square mi (47.7 square km)), which together harbored roughly 70 percent of the archipelago's fruit bats in the 1980s (Wiles et al. 1989). These two islands, which were estimated to support a total of 5,500 fruit bats in 1983, were estimated to have only 2,500 fruit bats in 2000; approximately a 45 percent decline since 1983 (Cruz et al. 2000d, 2000e). These declines may be related to severe habitat damage caused by feral ungulates (Cruz et al. 2000d, 2000e; Kessler 2000a; see discussion in Background, Habitat section).
On Anatahan, surveys identified about 3,000 fruit bats in 1983 (Wiles et al. 1989), 1,902-2,136 individuals in 1995 (Marshall et al. 1995; Worthington et al. 2001), and roughly 1,000 in 2000 (Cruz et al. 2000d; Kessler 2000a). In conjunction with the ungulate eradication project, fruit bats on Anatahan have been surveyed frequently since 2002. Aerial (helicopter) surveys were conducted in May 2002; February, March, April, August, October, and December 2003; and January, February, March, July, and September 2004. These surveys are Start Printed Page 1198performed over 2 days, with 4 hours spent over the island each day. Coverage of the island during each survey is complete. Fruit bat colonies are rapidly reconnoitered to verify known roost sites and identify new ones, colonies are counted and mapped, and individual bats in flight also are counted. After the volcanic eruption in May 2003, the island's state of devegetation facilitated accurate location of all colonies (C. Kessler, in litt. 2003, pers. comm. 2004c). In 2002 and early 2003, estimates of the island's bat population ranged from 950 to 1,250 (C. Kessler, in litt. 2003). Following Anatahan's volcanic eruption in May 2003, aerial surveys conducted in August, October, and December of 2003 yielded estimates of 350-700 bats, and in January and February of 2004, bat numbers were estimated at 500-600 and 550-650, respectively (C. Kessler, in litt. 2003, pers. comm. 2004c). Surveys in March, July, and September of 2004 yielded increased estimates of about 1,000-1,200 bats (C. Kessler, pers. comm. 2004c). This localized increase in fruit bat numbers over a short period of time (1 to 1.5 years) was concomitant with some vegetation recovery, and indicates that Anatahan's population may have reached its pre-eruption level, whether the source of the additional bats is immigration, recruitment of newly volant (flying) young, or both (see Summary of Factors Affecting the Species section).
On Pagan, fruit bat numbers were estimated at 2,500 in 1983 (Wiles et al. 1983), and at roughly 1,500 in 1999 and 2000 (Cruz et al. 2000e). On the third-largest northern island, Agrihan (18.3 square mi (mi2) (47.4 square km (km2)), results of surveys in 1983 and 2000 indicate that fruit bat numbers have been stable at about 1,000 individuals (Wiles et al. 1989; Cruz et al. 2000f).
The remaining northern islands with fruit bat populations, Maug, Asuncion, Alamagan, Guguan, and Sarigan, all are less than 5 square mi (13 square km) (Table 1), and harbor from 100 to 500 bats (Cruz et al. 2000a, b, c). Sarigan, the next island north of Anatahan, has been surveyed more frequently in recent years in conjunction with the ungulate eradication there. A 1997 survey of Sarigan estimated the population at 170 fruit bats, and a 1999 survey resulted in an estimate of 150-200 individuals (Wiles 1999). Surveys between 1983 and 2000 on Sarigan estimated populations of approximately 125-235 bats (Wiles et al. 1989; Fancy et al. 1999; Wiles and Johnson 2004). In 2001, surveys estimated 300-400 bats (Wiles and Johnson 2004). The observed increase on Sarigan may reflect a response to the recovery of forest vegetation after the eradication of feral goats and pigs from the island in 1998 (Zoology Unlimited 1998). As described above in the discussion of interislands movements, the increase in 2001 may also reflect immigration to Sarigan from Anatahan, 23 mi (37 km) to the south, as well as recruitment of newly volant young (Wiles and Johnson 2004). The potential for increase in fruit bat numbers on Sarigan is thought to be limited, however, by the island's small size (1.9 mi2 (4.9 km2)), the small extent of forest habitat (as described above, in the Habitat section), and the prevalence of monotypic stands of coconut, which provide only minimal forage habitat for fruit bats (Wiles and Johnson 2004; G. Wiles, Washington Department of Fish and Wildlife (formerly CNMI DFW), pers. comm. 2004).
Guam
On Guam, the sighting of fruit bats was considered to be “not * * * uncommon” in the 1920s (Crampton 1921). However, by 1931, bats were uncommon on Guam, possibly because of the introduction of firearms (Coultas 1931). Woodside (1958) reported that in 1958, the Guam population was estimated to number no more than 3,000, although the method used to make this estimate is not known (Utzurrum et al. 2004). This estimate had dropped by an order of magnitude, to between 200 and 750 animals by 1995, in part because of predation by the introduced brown treesnake (Wiles et al. 1995; Wiles 1996). During 1998, bat populations on Guam varied from an estimated low of 210-245 to a high of 910-980 bats (Wiles 1998), and in 1999, bat numbers ranged from an estimated low of 199-235 to a high of 327-371 (Wiles 1999). The most recent surveys on Guam put the bat population at fewer than 100 individuals (D. Janecke, in litt. 2003; A. Brooke, in litt. 2003). Predation by brown treesnakes on non-volant young probably prevents recruitment of juvenile bats on Guam (Wiles et al. 1995; Wiles 1996; G. Wiles, in litt. 2003).
Previous Federal Action
The Mariana fruit bat (Pteropus mariannus mariannus) was listed as endangered in 1984 on Guam (49 FR 33881). It was listed as a subspecies found only on Guam. More recent research over the years since this subspecies was listed indicates that Pteropus mariannus mariannus is not a subspecies endemic only to Guam but the Guam population is part of a subspecies including populations of bats on other islands that interact with each other (movement between islands). We believe that it is appropriate to list these bat populations in Guam and CNMI as one subspecies (63 FR 14641).
All the bat populations on Guam and in the CNMI are facing a number of threats, with most populations declining. We published a proposed rule on March 26, 1998 to reclassify the Mariana fruit bat on Guam from endangered to threatened and list all the bat populations on Guam and other CNMI islands as one subspecies throughout its range as threatened (63 FR 14641, 69 FR 30277).
We proposed to list the subspecies as threatened because we wanted to: (1) Simplify actions and expenditures. We could affect a downlisting for the population on Guam with little or no additional time and expense in conjunction with proposing to list the subspecies throughout its range, instead of taking a separate action to downlist the population on Guam; and (2) acknowledge a change in taxonomy. When we originally listed the population on Guam, we believed it to be a separate subspecies endemic only to Guam with a declining population and significant threats to it which merited endangered status. However, by including the other populations in the listing, we are evaluating a larger number of bats with a wider distribution, although threats to each population remain. Hence, we proposed threatened status for the entire population, instead of having one population as endangered and the others as threatened.
In that proposed rule, we included a detailed history of Federal actions completed prior to the publication of the proposal. The public comment period closed on May 11, 1998 (63 FR 14641) and was reopened from May 29, 1998, through July 10, 1998 (63 FR 29367) to accommodate requests for public hearings. We designated critical habitat for the Mariana fruit bat on Guam in a final rule published in the Federal Register on October 28, 2004 (68 FR 62944). Pursuant to a settlement agreement approved by the U.S. District Court for the District of Hawaii on August 21, 2002, we must make a final listing decision on the Mariana fruit bat and submit the final rule to the Federal Register by December 31, 2004. See Center for Biological Diversity v. Norton, Civil No. 99-00603 (D. Haw.).
Summary of Comments and Recommendations
In the proposed rule published on March 26, 1998 (63 FR 14641), we requested that all interested parties submit written comments on the Start Printed Page 1199proposal. We also contacted appropriate Federal, Territorial, and Commonwealth agencies, scientific experts and organizations, and other interested parties and invited them to comment on the proposal. Newspaper notices were published in the Marianas Variety (Saipan, CNMI) and Pacific Daily News (Guam), inviting general public comment and attendance at public hearings. We held public hearings on June 24, 1998, on Saipan and June 25, 1998, on Rota.
We reopened the public comment period on May 27, 2004 (69 FR 30277), to permit additional public review. In order to address any additional comments received during the reopened comment period, and meet the court order to submit to the Federal Register a final listing decision for the Mariana fruit bat no later than December 31, 2004, we reopened the comment period for 30 days, until June 28, 2004. The reopened comment period (and associated notifications in local media and via direct mailing) gave interested parties additional time to consider the information in the proposed rule and provide comments and new information.
During the first comment period in 1998, we received 13 written comments, including those submitted at the public hearings. During the reopened comment period in 2004, we received four additional written comments, including one from a Government of Guam agency, and one from a CNMI government agency. Several individuals or groups submitted comments in both the original and the reopened comment periods, or during hearings and later in writing. Of those comments received in 1998, eight opposed listing in the CNMI, one opposed listing in the CNMI and opposed downlisting on Guam, one opposed downlisting on Guam, one opposed downlisting on Guam but was in favor of listing in the CNMI, and one supported listing in the CNMI. In addition to several private citizens, the CNMI Governor, Director of the DFW, Rota DLNR Resident Director, Rota Mayor, and CNMI Senator Thomas P. Villagomez all opposed the proposal. The Air Force supported listing the fruit bat as threatened throughout the archipelago, but also stated that reclassification from endangered to threatened on Guam would be “misleading and confusing to the public,” and cited an article in the local press that misrepresented a temporary influx of fruit bats from Rota as an increase in the Guam population (Thomas Churan, Air Force, in litt. 1998; also see Issue 15, below). The Air Force also expressed its belief that the Mariana fruit bat is more susceptible to extirpation on Guam than in the CNMI because of the presence of the brown treesnake there, and recommended that the fruit bat retain its status as endangered on Guam (T. Churan, in litt. 1998). The Mariana Audubon Society supported listing all bats in the Mariana archipelago as endangered rather than threatened. Three of the four parties that submitted comments during the reopened comment period in 2004 supported the listing, including the DAWR. The CNMI DFW opposed the listing.
This final rule has been revised and updated to reflect the pertinent comments and information received during the comment periods. Comments of similar nature are grouped under a single issue. In addition, we considered and incorporated into the final rule all appropriate information obtained through the public comment period.
Peer Review
In 1998, in accordance with our peer review policy published on July 1, 1994 (59 FR 34270), we solicited opinions from four individuals who have expertise with the species and the geographic region where the species occurs, and are familiar with conservation biology principles. We received written comments from two experts and incorporated their information into the final rule. One peer reviewer described the threats posed to the bats on Guam by brown treesnake predation and habitat destruction by feral ungulates. This reviewer did not include any professional judgment about movement of bats between islands, but has published peer-reviewed literature containing information that supports interisland exchange. The other expert expressed agreement and knowledge that there is interisland exchange.
In 2004, we solicited additional scientific peer review of the proposed rule from eight specialists, including one of the two who provided peer review in 1998. Of these, five responded and provided additional factual information, including recent survey results, the impact of typhoons and illegal hunting on fruit bats in the southern islands, and recent genetic studies of other Pteropus species elsewhere in the Pacific. Reviewers also provided citations for literature, corrections on minor factual issues, and input on interpretation of the existing information.
One reviewer provided a synopsis of changes in fruit bat numbers over the past 10-20 years on individual islands in the archipelago and noted declines on Guam, Anatahan, and Pagan. This synopsis was based partly on the reviewer's own research and partly on the work of others. Based on 19 years of fruit bat research, surveys, and personal observations in the Mariana Islands while employed as a Senior Biologist with the Guam Division of Aquatic and Wildlife Resources, this reviewer (who also authored the original recovery plan for the Mariana fruit bat on Guam, agency reports, and numerous peer-reviewed research papers on the Mariana fruit bat (e.g., Wiles and Payne 1986; Wiles 1987a, b; Wiles et al. 1989; Wiles and Glass 1990; Wiles 1992; Wiles et al. 1995; Wiles and Johnson 2004) emphasized three major threats to Mariana fruit bats: illegal hunting (described as “chronic” on Rota), habitat destruction by feral ungulates, and brown treesnake predation. Another reviewer, a biologist who spent two years monitoring fruit bats on Rota and elsewhere in the CNMI for the CNMI DFW, provided specific information about firsthand observations and evidence of illegal hunting of fruit bats on Rota after Typhoon Pongsona, described reports received of numerous other illegal hunting, and provided survey information documenting post-typhoon decline in fruit bats on Rota and subsequent increase in numbers. Three reviewers, two of whom hold doctorates based on research on the biology and ecology of island fruit bats, and one of whom is currently conducting a graduate research project on fruit bats on Guam, expressed their professional opinions that anthropogenic disturbances such as illegal hunting and habitat loss are likely to be significant threats to the Mariana fruit bat, and that these disturbances are periodically exacerbated by severe storms.
Two reviewers cited their own observations and those of other workers that indicated likely interisland movements between Sarigan and Anatahan and between Rota and Guam, and another reviewer cited information collected by others indicating likely interisland movement in the archipelago. Three of the five reviewers provided information and professional opinion that supported our treating all fruit bats occurring in the Mariana archipelago as a single subspecies, Pteropus mariannus mariannus, as described in the proposed rule; the other two expressed concern about the possible occurrence of genetically isolated populations within the range of fruit bats in the Mariana Islands. Two reviewers expressed reservations about treating all fruit bats in the archipelago Start Printed Page 1200as one taxon without empirical data from genetic or radio-telemetry studies. However, one of these reviewers also described unpublished genetic research on fruit bats in Polynesia that indicates a lack of within-archipelago genetic structure in a widespread species that shares social and behavioral traits with the Mariana fruit bat.
Issue 1: The Service lacks adequate data to assess the population status of Mariana fruit bats. Comprehensive surveys are required to determine the status of Mariana fruit bats in the northern islands.
Our Response: In this case, we believe existing data are adequate to assess the overall status of the Mariana fruit bat. Subsequent to listing, two additional multi-island surveys of bats in the Mariana Islands have been conducted. One of these included six of the 10 northern islands (Cruz et al. 2000a-f) and yielded data comparable to those collected in 1983 by Wiles et al. (1989). The other conducted in 2001 (Johnson 2001) included all of the islands in the archipelago but employed methods that precluded direct comparison with other surveys. A conservative interpretation of these data indicate that bat numbers have declined on the two islands, which historically had large numbers of fruit bats in the archipelago.
Issue 2: The Service's evidence of bats moving between islands was inadequate or only anecdotal, and without empirical evidence of interisland movement, a determination that all fruit bats in the Mariana Islands belong to the same subspecies is premature. Fluctuations in bat numbers, particularly on Guam, may be caused by births.
Our Response: Evidence for the movement of bats between islands in the Mariana archipelago is discussed in the Background subsection above. The large fluctuations in the Guam bat population over a short period of time (Wiles 1998; A. Brooke, in litt. 2003) coupled with a low reproductive rate make it unlikely that changes in the Guam population reflect recruitment from births. Predation by brown treesnakes largely precludes the recruitment of young bats into the Guam population (Pierson and Rainey 1992; Wiles 1987a; G. Wiles in litt. 2003).
Issue 3: Long term survey data from Rota indicate natural fluctuations in fruit bat numbers on various timescales. Archipelago-wide surveys and the apparent decline they document may not account for these natural fluctuations.
Our Response: To date, we are aware of no analysis of survey data from Rota that: (1) Demonstrates a correlation between variation in fruit bat numbers and some other natural cycle, or (2) controls for the hunting and other human disturbance.
Issue 4: CNMI government agencies feel the Service overstated the illegal hunting problem, and stated that the CNMI DFW is instituting law enforcement reforms, and the CNMI government is committed to the enforcement of wildlife regulations. In contrast, most peer reviewers identified illegal hunting and lack of enforcement as a significant threat to the Mariana fruit bat, especially in the CNMI, and an official from Guam DAWR expressed concern that recruitment of immigrant bats to Guam is threatened by illegal hunting on Rota.
Our Response: We appreciate the CNMI DFW's commitment to law enforcement. We acknowledge that data on illegal hunting is difficult to obtain and assess, and that most of the information regarding illegal hunting is anecdotal. We have numerous documented observations and reports of illegal hunting incidents in the CNMI (e.g., Arnold Palacios, CNMI DWF, in litt. 1990; T. Eckhardt, Service, in litt. 1998; J. Esselstyn, pers. comm. 2004a; C. Kessler, pers. comm. 2004a). We address the threat to the Mariana fruit bats from illegal hunting in Factor B in the Summary of Factors Affecting the Species section.
Issue 5: The Service was selective in its presentation of the impacts of feral animals on Mariana fruit bats, presenting it in a poor light to justify listing. The Service did not consider the feral animal eradication project on Sarigan, and failed to note that the CNMI DFW has an existing federally funded program addressing feral animal damage (Feral Animal Monitoring and Management (Project No. W-1-R-1-11; Job number 2)).
Our Response: We have incorporated the results of the Sarigan Feral Animal Control Project (Zoology Unlimited 1998) into this final rule and discuss the threats posed to fruit bats by feral animals (see discussion in the Background section, and Factor A in the Summary of Factors Affecting the Species section). Although DFW's Feral Animal Monitoring and Management Program has included survey of feral animals on many of the northern islands and involvement in several other projects, current DFW projections indicate that sufficient funding will not be available to complete the eradication of feral ungulates from Anatahan, and lack of material support will prevent the implementation of plans for feral animal control in the CNMI (L. Williams, pers. comm. 2004).
Issue 6: Present CNMI Coastal Resources Management (CRM) and DLNR land use regulations adequately protect Mariana fruit bat habitat (limestone forest) from development, as exemplified by the modifications required for construction of the Rota Resort and Country Club. Habitat is also being protected through island-wide master planning and through implementation of habitat conservation plans (HCPs) on Saipan and Rota.
Our Response: We support the use of local land use regulations to promote the conservation of the Mariana fruit bat and its habitat. However, the best measure of their past effectiveness in protecting the Mariana fruit bat is the success of these regulations in maintaining the integrity of native limestone forest systems in the CNMI, particularly in the southern islands where development pressures are greatest. Direct and secondary effects of human activity continue to cause alteration of native forest areas despite these protections.
Through the Act's section 10 and HCP planning process, listed species may be lawfully taken and measures implemented to reduce activity impacts on the species and its habitat. Two HCPs are currently under development on CNMI and, if completed and implemented, should contribute to fruit bat conservation. The successful completion of these HCP projects in the CNMI is not sufficiently certain to consider them in making this listing decision. See our Policy for Evaluation of Conservation Efforts When Making Listing Decisions (PECE policy) (68 FR 15100, March 28, 2003).
Issue 7: The Service did not account for actions by the CNMI government to control the brown treesnake, thereby decreasing the threat of this factor to the Mariana fruit bat.
Our Response: We recognize that ongoing actions on Guam, Saipan, Tinian, and Rota are important and reduce the threat of accidental introduction of the brown treesnake. The U.S. Department of the Interior (DOI) Office of Insular Affairs (OIA), U.S. Department of Defense (DOD), USDA Wildlife Services, Service, Government of Guam, CNMI, and State of Hawaii are working together regionally to control brown treesnakes, particularly around transport centers (OIA 1999). The OIA and DOD actively fund research into methods of controlling snakes on Guam, in part to reduce the threat of introduction to other Pacific islands (OIA 1999). Both the CNMI DFW and Guam DAWR conduct brown treesnake public awareness educational campaigns Start Printed Page 1201consisting of school presentations, news releases, workshops, and poster/pamphlet distribution (Perry et al. 1996), and the CNMI maintains a snake reporting hotline (Nate Hawley, CNMI DFW, pers. comm. 2004a). In 1996, the CNMI became a signatory of the Memorandum of Agreement (MOA) between the governments of Hawaii, Guam, and the CNMI, and individual Federal government agencies concerned with brown treesnake eradication and control (DOI et al. 1993; DOI et al. 1996). This MOA commits the CNMI to a proactive brown treesnake program and allows the CNMI to apply for funding from the allotment of money appropriated by the U.S. Congress each year for brown treesnake control and eradication (OIA 1999).
Despite ongoing efforts, evidence exists that treesnakes are present on Saipan. A concrete barrier completed in 2004 at the commercial port on Saipan aids in the prevention of new introductions from Guam, but this barrier does not address the problem of the treesnakes already present on the island. The presence of brown treesnakes on Saipan poses a threat to the recovery of the fruit bat population there until the treesnakes are controlled throughout the island or are eradicated.
On Tinian, brown treesnakes, have been documented and are not thought to be established (Hawley 2002). The upcoming construction of a concrete snake barrier on Tinian will aid in the prevention of treesnake introductions to the island.
On Rota, two dead brown treesnakes were found in a cargo container in 1991, and in another, a live treesnake was sighted (N. Hawley, pers. comm. 2004a). The fence surrounding Rota's port was retrofitted with a snake barrier subsequent to the discovery of the two dead treesnakes, but damage and maintenance difficulties have resulted in deterioration of the barrier, and it was disassembled in 2002 (Gad Perry, U.S. Geological Survey-Biological Resource Division, in litt., 1998; N. Hawley, pers. comm. 2004b). CNMI DFW recommended replacing the fence with a concrete barrier around the cargo area; however, the barrier has not yet been constructed. These efforts were considered in the Summary of Factors Affecting the Species section below.
Issue 8: Existing regulations of the CNMI government are satisfactory for protecting the Mariana fruit bat so Federal listing is not necessary. The Mariana fruit bat is listed as threatened or endangered by the CNMI, and the Service was incorrect in stating that the CNMI lifted the moratorium on hunting of Mariana fruit bats. Therefore, the threat of legalized hunting is non-existent.
Our Response: We acknowledge that the CNMI has regulations protecting the Mariana fruit bat, but we have concluded that these regulations either do not contain sufficient protections or have not been adequately enforced to protect bat populations (see Factor D below).
In the proposed rule, we stated that the moratorium on the taking of Mariana fruit bats on all islands (Public Law 5-21, September 1977) had been lifted. We based this on a memo from the CNMI Assistant Attorney General for DLNR to our Law Enforcement (LE) office on Guam which stated that the hunting moratorium was no longer in effect (Richard Folta, Office of the Governor, Guam, in litt. 1996). In a subsequent letter to the Service, the Assistant Attorney General stated that the previous communication had been in error, and that the moratorium was still in effect (R. Folta, in litt. 1996). This new information has been incorporated into this final rule.
Issue 9: Listing the bat will not improve law enforcement, due in part, to the resource limitations of the Service's Division of Law Enforcement. No Service LE personnel are stationed in the CNMI, so the Service will be unable to enforce Federal regulations associated with the listing.
Our Response: The Service does have a wildlife inspector stationed in the Marianas who provides some enforcement of regulations associated with the Act. Declines in illegal fruit bat imports to Guam and the CNMI have been associated with the presence of LE personnel stationed on Guam and efforts of LE personnel based in Honolulu (Sheeline 1991; George Phocas, Service, pers. comm. 2004). We work in cooperative partnerships with Territorial, Commonwealth, State, local, and Federal agencies to further our interdiction and enforcement efforts. In the Mariana Islands, Service personnel are presently assisted by local customs officers, conservation officers, and quarantine officials in the enforcement of the Act. It is important to note that the Act provides an additional set of enforcement tools for the protection of listed species than are currently available for the fruit bat in the CNMI.
Issue 10: The listing of the Mariana fruit bat in the CNMI may result in severe harassment to the species.
Our Response: There has been no evidence to suggest that harassment of fruit bats is likely to occur as a result of listing. We understand that hunting of fruit bats takes place on a regular basis in the CNMI despite their protection under CNMI law, but all of the information we have received indicates that this hunting is motivated by local tradition, not by malicious intent in response to CNMI laws and regulations. Whatever the motivations for harassment or illegal hunting of Mariana fruit bats, their listing under the Act can provide additional protection through the enforcement of Federal law. In sum, we believe that the protections afforded to Mariana fruit bats by their being listed as threatened throughout their range will aid in their conservation and recovery.
Issue 11: Increased funding to the CNMI for endangered species recovery is unlikely. Listing the bat as threatened instead of endangered has the potential to restrict funding opportunities to conduct research and management because the Service's funding system places higher priority on species designated as endangered as compared to those listed as threatened.
Our Response: Under their cooperative agreement with us, DFW can apply for funding under section 6 of the Act for projects specifically related to Mariana fruit bat conservation. We do not categorically assign higher priority for funding or recovery actions to species that are listed as endangered over those that are listed as threatened.
Issue 12: Protection for the Mariana fruit bat on Farallon de Medinilla should come from the Service through the consultation process under section 7 of the Act. Listing the Mariana fruit bat in the CNMI will provide no additional protection with regard to military activities.
Our Response: Prior to the publication of this final rule, the Mariana fruit bat was not federally listed in the CNMI. Federal agencies, therefore, have not been required to consult on the effects of their actions in the CNMI on the fruit bat. Conversely, 30 days after the publication of this rule, the Mariana fruit bat becomes federally listed as threatened in the CNMI and throughout its range, and Federal agencies will be responsible for consulting with us when their activities may affect the fruit bat on Farallon de Medinilla or other islands in the CNMI.
Issue 13: The Service misinterpreted the data and conclusions of Morton (1996) in stating that military aircraft training activities on Guam cause or create the potential for abandonment of roosting areas.
Our Response: Current air traffic patterns and volume do not pose a threat. There is the potential for roost abandonment if air traffic patterns or volume increase significantly (Morton 1996). Significant changes could Start Printed Page 1202include more frequent departures and arrivals, and larger or noisier aircraft.
Issue 14: The rule is politically motivated, biased, based on assumptions and broad, unsubstantiated statements, speculative observations, and anecdotal evidence.
Our Response: We used the best scientific information available in our determination to list the Mariana fruit bat as threatened in the CNMI and reclassify from endangered to threatened on Guam. Threats to the Mariana fruit bat are documented in the Summary of Factors Affecting the Species section of this final rule. We did not rely solely on anecdotal information in making a decision to list this species as threatened. The rule includes citation to more than 70 published references, more than 40 scientific reports prepared for government agencies and universities, and numerous personal communications from scientists and others knowledgeable about fruit bats and the Mariana Islands and/or closely involved in natural resources management in the archipelago. The anecdotal information we did use is consistent with the body of scientific reports.
Issue 15: Some commenters felt that listing the Mariana fruit bat in the CNMI is justified, but many thought that reclassifying the fruit bat from endangered to threatened on Guam, and listing the fruit bat as threatened rather than endangered in the CNMI, was incorrect. Some of these commenters believe that reclassifying the Mariana fruit bat on Guam has already sent the wrong message to the public because media reports have misinterpreted the proposal as evidence of recovery. Some also expressed concern that reclassification of the fruit bat on Guam could undermine conservation funding. They suggest that the Service either leave the Guam population listed as endangered, or list all bats in the Mariana Islands as endangered rather than threatened.
Our Response: We define an endangered species as one which is in danger of extinction throughout all or a significant portion of its range. Threatened species are defined as those which are likely to become endangered within the foreseeable future throughout all or a significant portion of their range. Because we consider the fruit bats on all individual islands in the Mariana archipelago as part of a single, archipelago-wide subspecies, Pteropus mariannus mariannus, we now are evaluating a larger number of bats with a more widespread distribution than was evaluated for the original listing in 1984, which included only the fruit bat population on Guam. Listing Pteropus mariannus mariannus as threatened throughout its range, including bats in both the CNMI and Guam, retains an appropriate level of protection for this bat on Guam while increasing overall protection to the Mariana fruit bat throughout the Mariana Islands, and it does not undermine potential funding for fruit bat conservation on Guam.
Issue 16: The Service did not properly take into account the cultural importance of the Mariana fruit bat in its listing decision. For example, some commenters suggested that information from the document “Cultural Significance of Pacific Fruit Bats (Pteropus) to the Chamorro People of Guam” (Sheeline 1991) should have been incorporated into the proposed rule.
Our Response: We incorporated information contained in Sheeline (1991) into this final rule in the section Summary of Factors Affecting the Species, subsection B.
Issue 17: If listing occurs, the people of the CNMI deserve the same consideration that the Federal government has given to Native Americans, such as Alaskan natives, through inclusion of a provision to provide for limited take of Mariana fruit bats for cultural use.
Our Response: We recognize the importance of traditional values to native cultures. This is reflected in our close collaboration with agencies in the CNMI to develop HCPs. However, the Act specifically exempts only Alaskan natives from the take prohibitions if such take is primarily for subsistence purposes and meets certain other conditions (16 U.S.C.§ 1539 (e)), but subsistence take by other groups is not exempted by the Act.
Issue 18: One commenter stated that disease is the cause of decline of Mariana fruit bats on Rota.
Our Response: We are unaware of any evidence of disease affecting populations of Mariana fruit bats on Rota or elsewhere in the Mariana Islands.
Issue 19: The Service should clear up taxonomic questions surrounding the Mariana fruit bat and determine exactly how many taxa inhabit the Mariana Islands before listing is considered. Several peer reviewers expressed concern about the taxonomic uncertainties within western Pacific Pteropus, and that there may be more than one taxon endemic to the Marianas.
Our Response: Both the proposed and final rules address taxonomic questions in detail (see the Background subsection under SUPPLEMENTARY INFORMATION). If new information such as results from genetic studies of fruit bats in the Mariana Islands indicate the presence of additional subspecies, we will take appropriate action.
Issue 20: One commenter disagreed with the Service's proposed determination that designation of critical habitat for the Mariana fruit bat would not be prudent because the identification of specific locations as critical habitat would lead to increased illegal hunting, and would thus increase the threats to the species.
Our Response: Since publication of the proposed rule in 1998, several key court decisions have given us new guidance on making our “not prudent” critical habitat determinations. Furthermore, we now have designated critical habitat for the Mariana fruit bat on Guam (69 FR 62944). We have reexamined the prudency of designating critical habitat for the Mariana fruit bat based on these considerations and now determine that such a designation would be prudent. Our reasoning is presented in the Critical Habitat section below.
Issue 21: Why is the Service concerning itself with a listing priority tier 3/4 activity when other species are in greater need of attention? The Service published the proposed rule based on fiscal and timing reasons rather than biological reasons.
Our Response: This final rule was prepared under the terms of a Federal court-approved settlement agreement that stipulated we submit a final listing determination for the Mariana fruit bat to the Federal Register no later than December 31, 2004 (Center for Biological Diversity v. Norton, Civil No. 99-00603 (D. Haw.)).
Summary of Factors Affecting the Species
Section 4 of the Act and regulations (50 CFR part 424) promulgated to implement the listing provisions of the Act set forth the procedures for adding species to the Federal lists. A species may be determined to be an endangered or threatened species due to one or more of the five factors described in section 4(a)(1). These factors, and their application to the Mariana fruit bat (Pteropus mariannus mariannus) in the Mariana Islands are as follows:
A. The present or threatened destruction, modification, or curtailment of its habitat or range. Mariana fruit bats have been observed to feed on the fruits, flowers, and leaves of at least 22 plants, all but three of which are native to the Mariana Islands; fruit bats also have been documented to establish roosts primarily in mature Start Printed Page 1203native trees within landscapes dominated by native forest (Wiles 1983, 1987a). The Mariana fruit bat depends on native forest trees for food and colonial roost sites where mating, parturition, and other important social and biological functions take place. Although Mariana fruit bats have been observed to feed on cultivated food plants such as Artocarpus altilis and Carica papaya (Wiles 1987a), and have been observed to roost in Theobroma cacao (Glass and Taisacan 1988), nonnative plants make up a very small fraction of the resources used by the subspecies (Wiles 1987b; Worthington and Taisacan 1996) (see Habitat section above). The degradation and loss of native forest, therefore, deprives fruit bats of essential resources for survival and reproduction. The southern islands in the Mariana archipelago have lost most of their original native forest, primarily over several centuries of large-scale agriculture, growing human populations, economic development, and military activities (Bowers 1950; Fosberg 1960; see discussion). Few Mariana fruit bats occur today on Saipan, Tinian, and Guam, the islands that have sustained the greatest human disturbance and habitat loss.
Mariana fruit bats have evolved with, and are dependent for food and shelter on, trees and other plants that occur in native forests in the Mariana Islands. The degradation or loss of these forests is a key threat to the survival of this subspecies. The loss of native forests in the Marianas has various sources. The foraging of feral ungulates such as goats and pigs prevent forest regeneration because they eat ground-layer vegetation and seedlings of understory and canopy species; the rooting and stereotypical path-making of ungulates promote erosion and facilitate the invasion of native forests by alien plants (Marshall et al. 1995; Kessler 1997; Service 1998a,b). These invasive alien plants displace or smother native vegetation and prevent its regeneration (Kessler 2000b). In the southern islands of the CNMI and on Guam, where human influence has the longest continuous history, outright conversion of forests for agriculture or other development, as well as feral ungulates and alien plant species, historically has been a major source of loss of the Mariana fruit bat's forest habitat.
Throughout the archipelago, feral ungulates have caused severe damage to native forest vegetation by browsing directly on plants, causing erosion (Marshall et al. 1995; Kessler 1997; Service 1998a,b), and retarding forest growth and regeneration (Lemke 1992b). The remaining native forest habitat for fruit bats on many of these islands continues to be threatened by the fragmentation and degradation associated with feral ungulates. Mariana fruit bats are dependent on native plants for food and native forest for roost sites. Soil erosion and chronically retarded forest regeneration, the concomitant loss of native forests caused by the browsing and rooting of feral ungulates, and subsequent invasion by nonnative plant species, collectively represent a significant threat to fruit bats. These vegetation and landscape changes deprive the fruit bats of the native plant species on which they depend for food, shelter, and places to conduct their social activities. The diminished quality and extent of native forest thus leads to an associated reduction in the number of fruit bats that the remaining habitat is able to support. The northern islands, for the most part, have escaped the effects of millennia of continuous human settlement, WWII, and post war activities that caused extensive habitat loss and fragmentation of native forest habitat (see Table 2). However, the introduction of feral ungulates to some of these islands as recently as 40 years ago has resulted in rapid degradation and loss of native forest cover, notably on Anatahan and Pagan, two of the largest islands that have supported relatively large numbers of fruit bats (Kessler 1997, 2000a).
Island by Island Summary
Table 2 provides a synopsis of the numbers and status of fruit bats on each island in the archipelago.
Table 2.—Island Summary of Factors Affecting the Mariana Fruit Bat.
[See text for full discussion]
Island Area Mi2 (km2) Historical factors Key current factors Estimated fruit bat numbers and status Guam 212.0 (549.0) Hunting, habitat loss (development, agriculture, feral ungulates), brown treesnakes Brown treesnakes, habitat loss <100; declining.10 Rota 37.0 (95.7) Hunting, habitat loss (development, agriculture, feral ungulates) Hunting, habitat loss (development, feral ungulates) 1,100; fluctuating.9 Aguiguan 2.7 (7.0) Small island, feral ungulates Small island, feral ungulates Few individuals; possibly declining.8 Tinian 39.3 (101.8) Hunting, habitat loss (development, agriculture, feral ungulates) Habitat loss Low numbers; intermittent presence. 7 Saipan 47.5 (122.9) Hunting, habitat loss (development, agriculture, feral ungulates) Habitat loss, possibly brown treesnakes No colonies, few individuals.6 Farallon de Medinilla 0.8 (2.0) Small size, limited habitat, vegetation loss, erosion, fires Small size, limited habitat, vegetation loss, erosion, fires 2 fruit bats observed in 1996.5 Anatahan 12.5 (32.3) Feral ungulates Feral ungulates, invasive plants 1,000-1,200; decline since 1983; recovering from eruption.4 Sarigan 1.9 (5.0) Feral ungulates; little habitat Invasive plants; habitat limited to 72 ac (29 ha) 300-400; increasing since ungulate eradication.3 Guguan 1.5 (4.0) Small island, little habitat small island, little habitat 350; stable.2 Alamagan 4.3 (11.0) Feral ungulates Feral ungulates 200; possible increase since 1983.2 Pagan 18.4 (47.7) Feral ungulates Feral ungulates 1,500; decline since 1983.2 Agrihan 18.3 (47.4) Feral ungulates Feral ungulates (potential) 1,000; stable.2 Asuncion 2.9 (7.4) Small island; little habitat Small island; little habitat 400 1; stable or increasing. Start Printed Page 1204 Maug 0.8 (2.0) Small island; little habitat Small island; little habitat <251, unknown. 1 Wiles et al. 1989. 2 Cruz et al. 2000f (Agrihan); 2000e (Pagan); 2000b (Alamagan), 2000a (Guguan). 3 Wiles and Johnson 2004. 4 C. Kessler, pers. comm. 2004b. 5 T. Sutterfield, in litt. 1997. 6 L. Williams, pers. comm. 2004. 7 Krueger and O'Daniel 1999; Johnson 2001. 8 G. Wiles, pers. comm. 2004. 9 C. Kessler, pers. comm. 2004b. 10 A. Brooke, in litt. 2003. Habitat loss and degradation pose a significant threat to the Mariana fruit bat because it deprives them of foraging and sheltering resources that are necessary for survival and reproduction. The largest and most heavily populated southern islands in the archipelago have suffered the greatest habitat loss, primarily in the form of land conversion for agriculture, and military, commercial, and residential development and infrastructure. The most severely altered of these islands, Saipan, Tinian, and Guam, today support very few Mariana fruit bats. About half of the northern islands of the CNMI, including the three largest, harbor large populations of feral ungulates. These animals have caused severe damage to, and in parts, of some islands, a complete loss of native forest habitat.
Qualitative observations through time document increasing feral ungulate damage to native forest particularly on Pagan, Anatahan, and Alamagan (Wiles et al. 1989; Rice 1992; Kessler 1997, 2000a; Service 1998a, b; Zoology Unlimited 1998; Cruz et al. 2000b, d, e, f). Feral goats and pigs have been present on Anatahan for about 40 years, and observations indicate that, more recently, the severe ungulate damage on Anatahan apparently has been rapid. Thomas Lemke (Montana Department of Fish, Wildlife, and Parks, in litt. 1995) did not note significant erosion or large numbers of goats in the early 1980s. In 1992, Rice and Stinson (1992) did not see many feral animals but noted some areas where goat- and pig-caused damage was severe and warned that ungulate control was needed. In 1995, Marshall et al. (1995) observed many groups of goats, several pigs and widespread pig sign, and extensive loss of forest understory, devegetation, and erosion especially on the southern end of the island. Approximately 3,000 to 4,000 feral goats and 500 to 1,000 feral pigs were rapidly destroying the island's forests, and forest decline was directly associated with this decline in fruit bat numbers (Marshall et al. 1995; Kessler 2000a; Worthington et al. 2001). Photographic documentation provides evidence of rapid habitat alteration and loss between 1996 and 2000 (Kessler 2000a). Cruz et al. (2000d) described the feral ungulate damage they saw on Anatahan in 2000 as “an ecological disaster in progress.”
A program initiated in 2002 to eradicate goats from Anatahan has been resumed; however, not all goats have been removed and pigs are still present. Ground-based goat and pig eradication programs will have to wait until volcanic activity subsides (C. Kessler, pers. comm. 2004b). On Pagan, where domestic livestock was released from captivity in 1981, rapidly growing populations of feral goats, pigs, and cattle already have caused severe damage to native forest and conversion of forest to grassland (Kessler 1997; Cruz et al. 2000e). No projects are currently underway to remove ungulates or restore habitat on Pagan, Agrihan, or Alamagan. However, the eradication of feral goats from Sarigan (Zoology Unlimited LLC 1998) has been successful; it has resulted in some recovery of native vegetation and habitat for fruit bats on that island, although this habitat is limited in extent to roughly 72 acres (29 ha), and the island probably cannot support more than a few hundred fruit bats (Wiles and Johnson 2004).
The eradication of feral ungulates alone may not be sufficient to restore native habitat for fruit bats on the northern islands. The removal of grazing and browsing pressure apparently benefits invasive, alien plants, such as tangantangan and the vines Operculina ventricosa and Mikania micrantha, which are known to be significant threats to native vegetation on Pacific Islands (USDA 2004). These plants already have been observed to be increasing in abundance and alien vines are smothering other vegetation on Sarigan (where ungulates have been eradicated) and Anatahan (where goat numbers have been significantly reduced) (Kessler 2000a,b; C. Kessler, pers. comm. 2004b). Tangantangan forms dense, monotypic stands that exclude other vegetation, and the two climbing vines form mats that smother shrub and forest vegetation and prevent its regeneration. Without an effective control program, invasive alien vegetation may become a significant threat to fruit bat habitat on islands where ungulates have been removed.
DFW's Feral Animal Monitoring and Management Program has included surveys of feral animals on many of the northern islands. More recently, DFW's feral animal control efforts have included close involvement in the Sarigan goat eradication and subsequent monitoring, a 2001 survey of feral goats on Aguiguan, and vegetation monitoring and aerial control of feral goats on Anatahan (volcanic activity has interfered with plans to conduct ground-based goat and pig hunting on Anatahan) (L. Williams, pers. comm. 2004). These activities have been conducted with significant material and logistical assistance from the Navy and Service, and DFW is working with the Tinian Lands and Resources agency to increase feral goat hunting on Aguiguan. Currently, however, DFW anticipates that funding will not be available to complete the eradication of feral ungulates from Anatahan, and lack of material support will hinder realization of other existing plans for feral animal control in the CNMI (L. Williams, pers. comm. 2004).
The use of Farallon de Medinilla in the CNMI by U.S. armed forces as a bombardment range has limited vegetation, increased erosion that Start Printed Page 1205impedes regeneration of vegetation, and caused wildfires that destroyed habitat (Lusk et al. 1998). Together, these effects limit the habitat for fruit bats on this island.
The southern islands of the archipelago have historically been the most densely populated (Bowers 1950), and they have therefore sustained the greatest anthropogenic changes to the landscape and proportionally the greatest losses of Mariana fruit bats. Feral ungulates were well established by the 18th century. Tinian, for example, harbored as many as 10,000 cattle, and by mid-century the island's landscape included extensive pastureland and the remaining forest had no understory (Barrat 1988 in Stinson et al. 1992), and today the island has very few bats. Significant habitat conversion on these islands took place during the 20th century, and resulted from large-scale agriculture, human population growth, wholesale destruction from bombing (especially on Saipan and Tinian) during World War II, and the introduction of invasive alien plants (Bowers 1950; Fosberg 1960).
Between 1914 and 1944, extensive removal of native forests for development of sugar cane was greatly accelerated on the southern islands. Sugar cane fields covered almost all of Tinian and much of Aguiguan, Saipan, and Rota (Fosberg 1960). During and after World War II, military activities resulted in further dramatic reductions in fruit bat habitat on the southern islands. During this period, open agricultural fields and other areas prone to erosion on Saipan, Tinian, and Guam were seeded with tangantangan (Fosberg 1960). Tangantangan, which has a low to moderate stature and as described above grows in single-species stands with no substantial understory, provides no foraging resources or roost sites for fruit bats and is not suitable habitat for this species. Native forest cannot take root and grow where this alien tree has become established (Craig 1993), thus tangantangan effectively prevents regeneration of fruit bat habitat. After World War II, the extent of native forest remaining was estimated at 5 percent on Saipan, 2 percent on Tinian, 25 percent on Rota, and about 20 percent on Aguiguan (Bowers 1950). A report in 1986 estimated that Rota has 60 percent native forest cover (Engbring et al. 1986), but whether this indicates some forest recovery since World War II is not clear. Although there has been some regeneration of native forest on Rota, there has been little or none on Saipan or Tinian (Engbring et al. 1986). About 20 percent of the native forest persists on Aguiguan (Engbring et al. 1986) and these areas are occupied by feral goats.
On Guam, land development and feral ungulates have altered most of the native vegetation on the island. The pre-settlement extent of forest habitat on the island is unknown, but Guam was likely to have been densely forested prior to human settlement (Mueller-Dombois and Fosberg 1998). People first settled on Guam at least 3,500 years ago, and beginning in the 16th century, hundreds of years of foreign colonization and trade brought additional livestock and agricultural technology to Guam (and to the other southern islands in the archipelago) that resulted in increased landscape-scale habitat alteration (Fosberg 1960; Stone 1970). A U.S. Forest Service survey in 2002 estimated that approximately 63,830 ac (25,851 ha) or 48 percent of Guam's land area is under some type of forest (Donnegan et al. 2004). A map of forest and non-forest cover types on Guam produced by the same study clearly shows that the largest contiguous forest tracts are in northern Guam (Donnegan et al. 2004), on lands that belong primarily to the U.S. Air Force (Air Force) but that also include 50 ac (20 ha) that belong to the Service. Generally describing this pattern of contiguous forest in the north and fragmentation in the south, Donnegan et al. (2004) notes that “limestone soils in the north are covered with forest in areas not cultivated or urbanized,” and volcanic soils on the southern half of Guam are covered primarily by grassland, with some ravine forest occurring in sheltered and leeward sites.” Feral ungulates are abundant and widespread on the island and cause significant damage to the remaining native forest (Fosberg 1960; Stone 1970; A. Brooke, Service, pers. comm. 2004).
Lands owned by the Air Force at Andersen Air Force Base include the largest contiguous forested areas in northern Guam. Restricted access to Andersen Air Force Base, and to the Service's Guam National Wildlife Refuge at Ritidian Point, provides protection from poaching and other human disturbance of the single remaining fruit bat roost on Guam and significant foraging habitat in the northern part of the island. Other Federal, Government of Guam, and some private lands also have forested areas that include adequate habitat for bats (Wiles et al. 1995; 68 FR 62944).
Currently, the Air Force is proposing to expand development and operations at Andersen Air Force Base, and has initiated review of its proposal under the National Environmental Policy Act (NEPA) (Jeff Newman, Service, pers. comm. 2004). We do not have the details of the Air Force proposal at this time, nor do we know what effect this expansion may have on fruit bat habitat.
As on Guam, development and other human activities on Saipan and Tinian eliminated all but 5 percent of each island's native forest by 1982 (Engbring et al. 1986). On Saipan, the native forest has been replaced with mixed secondary growth forests, savanna grasslands, and dense thickets of tangantangan (Falanruw et al. 1989). Much of this habitat loss took place during World War II, when both islands were invaded (Baker 1946; Bowers 1950). The remaining forests on both islands continue to be threatened by planned development.
Rota experienced extensive agricultural development prior to World War II. The fact that Rota was not invaded and occupied during the war, combined with the island's rugged topography, resulted in Rota retaining a greater proportion of its native forest than Saipan or Tinian (Baker 1946). However, Rota's commercial and agricultural development poses a threat to the island's limestone forest. One 18-hole golf resort has been completed on Rota, another 1,025 ac (415 ha) are proposed to be developed into golf courses in the CNMI (CNMI Statistical yearbook 2001), and plans for additional large-scale development, together with smaller developments, continue to threaten the remaining limestone forest with destruction, fragmentation, and degradation.
In summary, loss of native forest habitat resulting from a variety of causes is a factor in the decline of the Mariana fruit bat. This loss restricts the availability of resources that fruit bats need to survive and reproduce, i.e., the native plants fruit bats feed on and the mature native forest trees where they roost, and thus limits the capacity of any island to support fruit bats. Saipan, Tinian, and Guam, the most severely altered islands, today harbor very few fruit bats. The ongoing loss and degradation of forest habitat in the archipelago continues to be a threat to the species.
B. Overutilization for commercial, recreational, scientific, or educational purposes. Mariana fruit bats have been used as food since humans first arrived on the islands (Lemke 1992a), and consumption of bats represents a significant cultural tradition. Social events and cultural status in the Mariana Islands are often enhanced by a variety of foods, and the fruit bat is a highly prized delicacy. Because of their scarcity, bats are often reserved for the elderly and other respected guests, and Start Printed Page 1206one bat may be shared among several people (Lemke 1992a). In a survey of Chamorros on Guam, 53 percent of the respondents indicated that they enjoyed eating fruit bat (Sheeline 1991). It is clear that the Marianas fruit bat is an important cultural symbol in the Mariana Islands, as 82 percent of the respondents to the same survey believed that fruit bats had cultural value. However, 85 percent of the respondents also believed people should stop hunting and eating fruit bats if such activity would lead to the species extinction (Sheeline 1991).
Traditionally, fruit bats were captured with limited success using nets, traps, thorny branches on poles, or stone projectiles (Lemke 1992a). Today, bats are mostly taken with shotguns fired at roosting and feeding sites or along flyways. It is important to note that gregarious fruit bats such as the Mariana fruit bat are particularly vulnerable to hunting at their roost sites. One shotgun blast may kill several bats or knock them to the ground, and a successful raid can glean up to 50 bats (Wiles 1987b; Lemke 1992a). Once fruit bats are on the ground, they are unable to take flight and are essentially helpless. Hunting at nursery colonies can also result in direct mortality and abandonment of infant and juvenile bats (Lemke 1992a). In Sheeline's (1991) survey, 45 percent of the respondents believed overhunting was the primary reason for the decline of fruit bats on Guam.
From 1975 to 1981, prior to listing of the Mariana fruit bats as endangered on Guam (49 FR 33881), approximately 15,800 fruit bats were shipped to Guam from Rota and Saipan for human consumption (Wiles and Payne 1986). This number could be twice the total number of Mariana fruit bats in existence today. During the last two decades, thousands of fruit bats have been shipped annually into the Mariana Islands from other Pacific islands for human consumption. Most of these shipments were the subspecies Pteropus mariannus pelewensis from the Republic of Palau. A single fruit bat can sell for U.S. $50-$75 in the CNMI (Worthington and Taisacan 1996; C. Kessler, in litt. 2003), where hunting of fruit bats has been illegal since 1977.
Overhunting, along with habitat loss, is cited as a causal factor in the initial fruit bat declines on Guam, Saipan, and Tinian (Perez 1972; Wheeler 1980; Wiles 1987b). Hunting-related declines on Guam, where hunting of fruit bats had been illegal since 1973, led to Federal listing as endangered on Guam in 1984 (49 FR 33881). Numerous documented reports indicate that hunting continues to be a threat to the Mariana fruit bat (Glass and Taisacan 1988; Lemke 1992b; Marshall et al. 1995; Worthington and Taisacan 1996; Stan Taisacan, CNMI DFW, pers. comm. 1997a, b; Rainey 1998; Nathan Johnson, CNMI DFW, pers. comm. 2000; G. Wiles, in litt. 2003; J. Esselstyn, pers. comm. 2004a; C. Kessler, pers. comm. 2004a; Arlene Pangelinan, Service, pers. comm. 2004). This long history of observations by CNMI biologists on Rota indicates some level of illegal hunting is occurring.
Illegal hunting of fruit bats on the northern islands is occasionally reported. In 1996, it was reported to be an increasingly significant problem in the CNMI (Worthington and Taisacan 1996). On Anatahan, which lies only 94 mi (151 km) from heavily-populated Saipan, remains of recently cooked fruit bats were found in the main campsite area in 1995 (Marshall et al. 1995). Also in 1995, a team of DFW biologists on the island observed residents of Anatahan cooking and eating fruit bats (Ann Marshall, Service (formerly CNMI DFW), pers. comm. 2004).
In 1998, 14 poached Mariana fruit bats were confiscated from a CNMI vessel returning from the northern islands (T. Eckhardt, in litt. 1998), and illegal hunting of Mariana fruit bats was reported on the island of Sarigan (Zoology Unlimited LLC 1998). On Pagan, 7 recently expended .410 (very small bore) shotgun shells were found in 1999, 4 more were found in 2000, and a .410 shell and fresh remains of cooked fruit bat were found during a helicopter refueling stop in 2001 (Cruz et al. 2000e; Johnson 2001). This size of ammunition is too small for hunting goats, pigs, or other ungulates, but can be used for birds as well as fruit bats. That expended shells were found in conjunction with fruit bat remains points to this ammunition being used to hunt fruit bats. Although the frequency of illegal hunting in the Northern Islands is likely low and difficult to quantify, this evidence supports that it does occur.
In 1987, between three and eight bats were reported to be illegally hunted from a small colony on Saipan (Glass and Taisacan 1988). In 1997, there was a report of nearly 90 bats that were illegally hunted on Tinian from a colony that roosted on the island briefly (Tim Sutterfield, Navy, pers. comm. 1998). Following supertyphoon Roy in 1988, defoliation and other damage caused by the storm forced bats on Rota to forage during the day in areas close to human habitation (Lemke 1992b; see Factor E). As a result, extensive illegal hunting occurred, contributing to a reduction of the total Rota population by more than half (A. Palacios, in litt. 1990). Although bat numbers on Rota had risen again to more than 2,000 before supertyphoon Pongsona in December 2002, the population again declined by more than half following this storm. With illegal hunting as a contributing factor, this decline was documented by monthly surveys conducted by the same individuals using the same techniques (evening colony departures, direct colony counts, and searches for solitary bats). These surveys yielded estimates of fewer than 750 animals for most of the 15 months following the supertyphoon (J. Esselstyn, in litt. 2003, pers. comm. 2004b). Similar sharp increases in hunting of fruit bats following severe storms has been documented in American Samoa as well as in the Mariana Islands (Craig et al. 1994; see Factor D).
Continued illegal hunting on Rota is reported to diminish the fruit bat population's rate of recovery to pre-storm abundance as observed by CNMI biologists (Worthington and Taisacan 1996). Hunter interviews indicated that hunting pressure on fruit bats has increased by roughly 31 percent in the year since Pongsona (J. Esselstyn, pers. comm. 2004a). As recently as July 2004, we received reports from members of the community on Rota that one or more illegal hunting incidents in June and July killed at least 40 fruit bats, resulting in the abandonment of the largest colony on the island, and another smaller colony had been abandoned as well (C. Kessler, pers. comm. 2004a). On August 22-23, 2004, 21 months after supertyphoon Pongsona, supertyphoon Chaba hit the Mariana Islands, and Rota sustained severe damage. Information that we received indicates that this storm may have defoliated as much as 60 to 75 percent of the island (A. Pangelinan, pers. comm. 2004). Fruit bats were seen foraging near and on the ground; frequent gun-shots and cooking of fruit bats were noted following the storm (A. Pangelinan, pers. comm. 2004). This level of illegal hunting, characteristic of the post-typhoon period, taking place again so soon after previous typhoons, is likely to compound the effects.
C. Disease or predation. The brown treesnake, which has caused the extinction of several bird species on Guam (Savidge 1987), is probably responsible for the lack of recruitment in the single remaining Mariana fruit bat colony on that island (Wiles 1987a; Pierson and Rainey 1992). Although only two cases of treesnake predation on Guam bats have been reported (Wiles 1983), the brown treesnake is Start Printed Page 1207considered capable of preying on non-volant young bats at their roosts (Service 1990). Wiles (1987b) and Wiles et al. (1995) suggested that the nocturnal brown treesnake will prey on young bats that have become too large to be carried by their mothers and are left at the roosts at night. In 1982, 46.6 percent of all juvenile Mariana fruit bats counted in northern Guam were judged to be in this size class, but between 1984 and 1986, after brown treesnakes had spread into the area, no bats of this size class were observed (Service 1990).
The brown treesnake was accidentally introduced to Guam between 1945 and 1952, probably in ship cargo (Rodda et al. 1992). By 1986, the treesnake had reached the extreme northern end of the island (Savidge 1987), and was probably present throughout the island. Because of a variety of historical and ecological factors associated with the treesnake, along with Guam's location and role as a major transportation hub in the Pacific, the probability is high that human activities will disperse brown treesnakes from Guam to other Pacific islands (Fritts 1988).
Reports of treesnakes found in the CNMI, especially on the island of Saipan, have increased since 1982 (Brown Treesnake Control Plan 1996). As of July 2004, on Saipan there have been 62 credible brown tree snake sightings resulting in the capture of 11 live brown treesnakes (N. Hawley, pers. comm. 2004a). The frequency of treesnake sightings on Saipan reported from 1982 through 2004 indicates that brown treesnakes are present on the island (Brown Treesnake Control Plan 1996; N. Hawley, pers. comm. 2004a) leading to increased predation risks. No reports of brown treesnakes exist from other islands in the archipelago.
D. The inadequacy of existing regulatory mechanisms. Prompted by severe declines in fruit bat numbers, the CNMI legislature in 1977 passed a moratorium on the taking of fruit bats on all islands (Pub. L. 5-21, September 1977). However, no agency possessed authority to enforce the law until the CNMI DFW was created in 1981 (Lemke 1992a). The bat has since been listed as threatened or endangered (the CNMI makes no specific distinction between the threatened and endangered categories) by the CNMI government on Rota, Saipan, Tinian, and Aguiguan (CNMI 1991). The CNMI's designation of threatened or endangered species does not include prohibition on take (K. Garlick, Service, in litt. 1997) or any other protection (A. Palacios, in litt. 1990; Worthington and Taisacan 1996). However, current CNMI hunting regulations (Part 4, Section 10.7.i (Commonwealth Register Vol. 23, August 16, 2001, p. 18266)) prohibit the hunting, killing, or possessing of threatened, endangered, and protected species. DFW has statutory authority to promulgate and enforce such regulations to protect fruit bats and impose fines for violations (L. Williams, pers. comm. 2004).
However, it has been reported that there is little enforcement of the hunting ban, and few investigations or convictions have taken place (Lemke 1992a; Tina de Cruz, CNMI DFW, pers. comm. 2003). In addition, following supertyphoon Pongsona, a CNMI biologist on Rota reported observing at least two individuals illegally hunting fruit bats from a colony, received a report from a conservation officer of five hunting parties in the vicinity of the same colony, and received anecdotal reports of illegal hunting at least two additional colonies, but no one was apprehended or cited for illegal hunting (J. Esselstyn, in litt. 2003). Also, although the Mariana fruit bat season is currently closed under DFW regulations (CNMI 1986), the DFW has, in the past, authorized special bat hunts on Rota and Anatahan. In light of this, there is the possibility that DFW will authorize special bat hunts on Rota in the future.
The Mariana fruit bat also is listed as an endangered species by the Government of Guam and take is prohibited under this designation (Wiles 1982). On Guam, the bat is legally protected from hunting by its endangered status under U.S. and Guam laws, and it is physically protected because the primary colony is in a remote location on Air Force lands where access is restricted.
On October 22, 1987, Pteropus mariannus was included in Appendix II of the Convention on International Trade in Endangered Species (CITES), a treaty established to prevent international trade that may threaten the survival of plant and animal species. Continuing declines in fruit bat populations resulted in the reclassification of P. mariannus to Appendix I of CITES on January 18, 1990, as well as the listing of all other species of Pteropus under Appendix II of CITES (except those species already listed under Appendix I), in an effort to control shipments and to encourage exporting countries to conserve their bat populations. All subspecies of P. mariannus are now protected under Appendix I of CITES (50 CFR part 23).
Generally, both import and export permits are required from countries before a CITES Appendix I species may be shipped, and Appendix I species may not be imported for primarily commercial purposes. CITES permits may not be issued if the export will be detrimental to the survival of the species or if the specimens were not legally acquired. However, CITES does not itself regulate take or domestic trade of wildlife between islands in the Mariana archipelago, as they are not separate countries.
The Republic of Palau became subject to the CITES restrictions for trade with the Mariana Islands when it established its independence from the United States in October 1994. However, small numbers of fruit bats from Palau continue to be intercepted in the Mariana Islands (G. Phocas, pers. comm. 2004; J. Esselstyn, pers. comm. 2004c). Reports suggest that Appendix I fruit bat species continue to be smuggled into the Mariana Islands from points as diverse as Samoa, the Federated States of Micronesia, and the Philippines, although with far less frequency than in the 1980s. An integrated approach of regulation, enforcement, and outreach, began in the 1990s by the Service on Guam, sought out a variety of agencies and other parties. Importation records suggest that these efforts, along with an export inspection program in Palau, may have slowed a region-wide harvest of Pteropus fruit bats; importation into the Marianas has dropped from tens of thousands each year to small “personal” shipments (G. Phocas, pers. comm. 2004). Experts and Federal law enforcement personnel are concerned that the demand for fruit bats will remain high, and that the reduction of international smuggling may have increased illegal hunting pressure on Rota and the northern islands (Worthington and Taisacan 1995; Wiles 1996; G. Phocas, pers. comm. 2004). Despite existing regulatory mechanisms for the protection of the Mariana fruit bat, illegal hunting and international trafficking in fruit bats continues to occur leading to reductions in fruit bat populations.
E. Other natural or manmade factors affecting its continued existence. Military training activities in areas used by fruit bats could disrupt the behavior of these bats. In general, military training activities including live-fire exercises and aircraft overflights, in or near areas on any of the islands that support fruit bats, are likely to disrupt fruit bat behavior and may result in mortalities. A study of the effects of aircraft overflights on the Mariana fruit bat at Andersen Air Force Base, Guam, found that current levels of air traffic appear to be within levels that are tolerable to the colony at Pati Point. Higher levels of aircraft traffic, particularly low-level field carrier Start Printed Page 1208landing practices (FCLPs), would have the potential to cause partial or complete abandonment of the Pati Point roost (Morton 1996). Nocturnal FLCPs and other air traffic pose an even greater risk to fruit bats because animals are in the air, traveling between the roost and various foraging areas at night; under these circumstances it is possible that low-flying aircraft may even strike bats (Morton 1996). An increase in air traffic at Andersen Air Force Base has been proposed and is currently under NEPA review (J. Newman, pers. comm. 2004).
The small number of Mariana fruit bats remaining on some islands (e.g., Guam, Saipan, and Aguiguan) may place bats on these islands at risk of extirpation from natural disturbances, environmental changes, and other chance events to which small populations typically are vulnerable (Meffe and Carroll 1997). Typhoons, in particular, could eliminate bats on one or more of these islands, although with sufficient time and suitable remaining habitat, these islands could be recolonized by immigrants.
Typhoons can drastically reduce or alter forested areas that constitute fruit bat habitat; under natural or prehistoric conditions, the size of fruit bat populations and the extent of forest habitat were sufficient for the species to coexist with this natural disturbance. Today, however, such storms can exacerbate the anthropogenic pressures on the Mariana fruit bat. In 1988, supertyphoon Roy defoliated or altered almost all of the forested areas on Rota (Fancy and Snetsinger 1996). Another typhoon that hit the northern island of Maug in 1981 also had similar devastating effects on fruit bat habitat (Lemke 1992b). Rota was hit hard most recently by supertyphoons Pongsona (December 2002) and Chaba (August 2004), and the island's forest habitat was further damaged.
The impacts of severe storms on fruit bat habitat can change fruit bat foraging and roosting behavior by temporarily modifying forest structure, changing tree species composition (by facilitating encroachment of nonnative species), and decimating important food resources (Lemke 1992b). The latter condition is particularly important, because when typical food resources are not available, fruit bats may seek forage in places and at times that increase their vulnerability to illegal hunting (Craig et al. 1994; Pierson et al. 1996). There is no evidence that direct mortality of fruit bats caused by the supertyphoons Roy and Pongsona was significant (Lemke 1992b; J. Esselstyn, in litt. 2003). However, defoliation and other damage caused by storms forces bats to forage during the day in areas close to human habitation (Lemke 1992b). Fruit bats were illegally hunted on Rota after both Roy and Pongsona, contributing to an observed reduction in numbers (A. Palacios, in litt. 1990; J. Esselstyn, in litt. 2003, in litt. 2004b).
The northern islands of the CNMI were formed by volcanic activity on the Mariana trench. This trench is a subduction zone, where one tectonic plate of the Earth's lithosphere is moving beneath another. The northern islands thus all have the potential for volcanic activity, and eruptions are another natural disturbance that may alter fruit bat habitat in the northern islands. Pagan last erupted in 1981 and a lava flow covered a part of the island. Anatahan erupted in May 2003, and much of the island was denuded. As described previously in “Status of CNMI Northern Islands,” the fruit bat population on Anatahan declined from more than 1,000 prior to the eruption to 350-450 individuals in December of 2003 (C. Kessler, in litt. 2003), but the population appeared to be recovering by March 2004, when more than 1,000 bats were recorded (C. Kessler, pers. comm. 2004c). Few humans have visited the island since the May 2003 eruption, and illegal hunting there is thus unlikely to have confounded the response of Anatahan's bat population to this natural disturbance.
Conclusions
The loss of native forest, predation (on Guam and possibly on Saipan) by the brown treesnake, and illegal hunting (especially on Rota) are the most significant threats to the survival of this species. Feral ungulates continue to severely degrade fruit bat forest habitat on some of the northern islands. Few bats occur on Guam, Saipan, Tinian, Aguiguan, and Maug, and such small numbers are highly vulnerable to severe storms and other climate events that can effect the vital rates of a population and to biotic changes within a population (such as sex ratio, age structure, and other demographic parameters) that can affect reproduction and survival of individual animals (Meffe and Carroll 1997). A significant number of fruit bats persist on Rota, and numbers there have shown some rebound following a documented decline after Typhoon Pongsona. Rota's fruit bats remain at risk from illegal hunting and loss of forest habitat. Fruit bats from Rota are believed to move among the southern islands, and this population is considered to be critical to the long-term stability of fruit bats in the Mariana Islands (Wiles and Glass 1990). The brown treesnake adversely impacts recruitment of bats on Guam, and there have been a significant number of sightings of this predator on Saipan. Therefore, listing the Mariana fruit bat as threatened in the CNMI is warranted.
The evidence of interisland movement between the islands of the Mariana archipelago (Wiles and Glass 1990; Wiles and Johnson 2004) indicates that the Mariana fruit bats in the Mariana Islands be viewed and managed as one taxon. In developing this rule, we have assessed the best scientific and commercial information available regarding the past, present, and future threats faced by the Mariana fruit bat. Based on this information, we believe that it is biologically appropriate to consider fruit bats on each island on Guam and the CNMI as part of one population, and the appropriate action is to, reclassify the Mariana fruit bat from endangered to threatened on Guam, and list the Mariana fruit bat as threatened throughout its range in the CNMI.
Critical Habitat
Critical habitat is defined in section 3 of the Act as: (i) The specific areas within the geographical area occupied by a species, at the time it is listed in accordance with the Act, on which are found those physical or biological features (I) essential to the conservation of the species, and (II) that may require special management considerations or protection, and (ii) specific areas outside the geographical area occupied by a species at the time it is listed in accordance with the provisions of section 4 of the Act, upon a determination by the Secretary that such areas are essential for the conservation of the species. “Conservation” means the use of all methods and procedures needed to bring the species to the point at which protection under the Act is no longer necessary.
Section 4(a)(3) of the Act and implementing regulations (50 CFR 424 part 12) require that, to the maximum extent prudent and determinable, we designate critical habitat at the time the species is determined to be threatened or endangered. Our implementing regulations (50 CFR 424.12(a)) state that the designation of critical habitat is not prudent when one or both of the following situations exist: (1) The species is threatened by taking or other human activity, and identification of critical habitat can be expected to increase the degree of threat to the species, or (2) such designation of critical habitat would not be beneficial to the species.
On October 15, 2002, we published a proposed rule designating critical Start Printed Page 1209habitat for the Mariana fruit bat and two other species on Guam (67 FR 63738). The final rule was published on October 28, 2004 (68 FR 62944).
Available Conservation Measures
Conservation measures provided to species listed as endangered or threatened under the Act include recognition, recovery actions, requirements for Federal protection, and prohibitions against certain activities. Recognition through listing results in public awareness and encourages conservation actions by Federal, State, Tribal, and local agencies, non-governmental conservation organizations, and private individuals. The Act provides for possible land acquisition and cooperation with States and requires that recovery actions be carried out for listed species. Recovery planning and implementation, the protection required by Federal agencies, and the prohibitions against certain activities involving listed animals are discussed, in part, below.
The primary purpose of the Act is the conservation of endangered and threatened species and the ecosystems upon which they depend. The ultimate goal of such conservation efforts is the recovery of these listed species, so that they no longer need the protective measures of the Act. Subsection 4(f) of the Act requires the Service to develop and implement plans for the conservation of endangered and threatened species (“recovery plans”). The recovery process involves halting or reversing the species' decline by addressing the threats to its survival. The goal of this process is to restore listed species to a point where they are secure, self-sustaining, and functioning components of their ecosystems, thus allowing delisting.
Recovery planning, the foundation for species recovery, includes the development of a recovery outline shortly after a species is listed, and later, preparation of draft and final recovery plans, and revision of the plan as significant new information becomes available. The recovery outline—the first step in recovery planning—guides the immediate implementation of urgent recovery actions, and describes the process to be used to develop a recovery plan. The recovery plan identifies site-specific management actions that will achieve recovery of the species, measurable criteria that determine when a species may be downlisted or delisted, and methods for monitoring recovery progress. Recovery teams, consisting of species experts, Federal and State agencies, non-government organizations, and stakeholders, are often established to develop recovery plans. When completed, a copy of the recovery outline, draft recovery plan, or final recovery plan will be available from our Web site (http://endangered.fws.gov), or if unavailable or inaccessible, from our office (see FOR FURTHER INFORMATION CONTACT section). We issued a recovery plan for the fruit bat on Guam (Service 1990); this listing rule will trigger a new recovery planning process for the Mariana fruit bat.
Implementation of recovery actions generally requires the participation of a broad range of partners, including other Federal agencies, states, non-governmental organizations, businesses, and private landowners. Examples of recovery actions include habitat restoration (e.g., restoration of vegetation), research, captive propagation and reintroduction, and outreach and education. The recovery of many listed species cannot be accomplished solely on Federal lands. To achieve recovery of these species requires cooperative conservation efforts on private lands as many occur primarily or solely on private lands.
The funding for recovery actions can come from a variety of sources, including Federal budgets, State programs, and cost share grants for non-Federal landowners, the academic community, and non-governmental organizations. In addition, pursuant to section 6 of the Act, we would be able to grant funds to the CNMI and Government of Guam for management actions that promote the protection and recovery of the Mariana fruit bat. Information on our grant programs that are available to aid species recovery can be found at: http://endangered.fws.gov/grants/index.html. In the event that our internet connection is inaccessible, please check www.grants.gov or check with our grant programs contact at U.S. Fish and Wildlife Service, Ecological Services, 911 NE 11th Avenue, Portland, OR 97232-4181 (telephone 503/231-6241; facsimile 503/231-6243).
Please let us know if you are interested in participating in recovery efforts for the Mariana fruit bat. Additionally, we invite you to submit any further information on the species whenever it becomes available and any information you may have for recovery planning purposes (see FOR FURTHER INFORMATION CONTACT section).
Section 7(a) of the Act, as amended, requires Federal agencies to evaluate their actions with respect to any species that is proposed or listed as endangered or threatened, and with respect to its critical habitat if any is being designated. Regulations implementing this interagency cooperation provision of the Act are codified at 50 CFR part 402. Section 7(a)(2) requires Federal agencies, including the Service, to ensure that activities they authorize, fund, or carry out are not likely to jeopardize the continued existence of a listed species or to destroy or adversely modify its critical habitat if any has been designated. If a Federal action may affect a listed species or its critical habitat, the responsible Federal agency must enter into formal consultation with us.
Federal agency actions that may require consultation for the Mariana fruit bat include, but are not limited to actions within the jurisdiction of the U.S. Army Corps of Engineers, Federal Emergency Management Agency, Federal Highways Administration, Federal Aviation Administration, U.S. Department of Housing and Urban Development, Natural Resources Conservation Service, and branches of the DOD. Parts of Guam, Tinian, and Farallon de Medinilla are used as, or are under consideration for use as, military bases or training areas by U.S. armed forces. Parts of Guam are federally owned by the DOD and Service, and three-fourths of Tinian and all of Farallon de Medinilla are leased by the Navy. Activities on these lands will trigger consultation under section 7 if they may affect the Mariana fruit bat. Federally supported activities that could affect the Mariana fruit bat or its habitat in the future include, but are not limited to, the following: Helicopter over-flights, bombardment and live-fire exercises, troop movements, agricultural projects, and construction or improvement of roads, airports, firebreaks, radio towers, and housing and other buildings.
The Act and its implementing regulations set forth a series of general prohibitions and exceptions that apply to all endangered and threatened wildlife. The prohibitions of section 9(a)(2) of the Act, implemented by 50 CFR 17.21 and 17.31 for endangered and threatened species, make it illegal for any person subject to the jurisdiction of the United States to take (includes harass, harm, pursue, hunt, shoot, wound, kill, trap, or collect; or attempt any of these), import or export, ship in interstate commerce in the course of a commercial activity, or sell or offer for sale in interstate or foreign commerce any listed species. It is also illegal to possess, sell, deliver, carry, transport, or ship any such wildlife that has been taken illegally. Further, it is illegal for any person to attempt to commit, to solicit another person to commit, or to cause to be committed, any of these acts. Start Printed Page 1210Certain exceptions apply to our agents and State conservation agencies.
Permits may be issued to carry out otherwise prohibited activities involving threatened animal species under certain circumstances. Regulations governing permits are codified at 50 CFR 17.22 and 17.23. Such permits are available for scientific purposes, to enhance the propagation or survival of the species, and/or for incidental take in connection with otherwise lawful activities. For threatened species, permits are also available for zoological exhibition, educational purposes, or special purposes consistent with the purposes of the Act. Requests for copies of the regulations regarding listed wildlife and inquiries about permits and prohibitions may be addressed to U.S. Fish and Wildlife Service, Endangered Species Permits, 911 NE 11th Avenue, Portland, OR 97232-4181.
It is our policy, published in the Federal Register on July 1, 1994 (59 FR 34272), to identify to the maximum extent practicable at the time a species is listed, those activities that would or would not constitute a violation of section 9 of the Act. The intent of this policy is to increase public awareness of the effect of this listing on proposed and ongoing activities within the range of the species. We believe that, based on the best available information, that most scientific or recreational activities (other than capturing or hunting fruit bats) that do not damage habitat within forested areas that support Mariana fruit bats would not likely result in violations of section 9.
We believe the following activities could potentially result in a violation of section 9, but possible violations are not limited to these actions alone:
(1) Unauthorized collecting, handling, possessing, selling, delivering, carrying, or transporting of the species, including import or export across State lines and international boundaries;
(2) Intentional introduction of exotic species that compete with or prey on bats, such as the introduction of the predatory brown treesnake to islands that support bat colonies;
(3) Activities that disturb Mariana fruit bats at roost sites and feeding areas; and
(4) Unauthorized destruction or alteration of forested areas that are required by the bats for foraging, roosting, breeding, or rearing young.
We do not consider these lists to be exhaustive, and provide them as information to the public. You should direct questions regarding whether specific activities would constitute a violation of section 9 to the Pacific Islands Fish and Wildlife Office (see FOR FURTHER INFORMATION CONTACT section). Requests for copies of the regulations concerning listed animals and general inquiries regarding prohibitions and permits may be addressed to the U.S. Fish and Wildlife Service, Endangered Species Permits, 911 N.E. 11th Avenue, Portland, OR 97232-4181 (telephone 503/231-2063; facsimile 503/231-6243).
National Environmental Policy Act
We have determined that environmental assessments and environmental impact statements, as defined under the authority of the National Environmental Policy Act of 1969, need not be prepared in connection with regulations adopted pursuant to section 4(a) of the Act. We published a notice outlining our reasons for this determination in the Federal Register on October 25, 1983 (48 FR 49244).
References Cited
A complete list of all references cited herein is available upon request from our Pacific Islands Fish and Wildlife Office (see FOR FURTHER INFORMATION CONTACT section).
Author
The primary author of this document is Holly Freifeld, Pacific Islands Fish and Wildlife Office (see ADDRESSES section).
Start List of SubjectsList of Subjects in 50 CFR Part 17
- Endangered and threatened species
- Exports
- Imports
- Reporting and recordkeeping requirements
- Transportation
Regulation Promulgation
Start Amendment PartAccordingly, we amend part 17, subchapter B of chapter I, title 50 of the Code of Federal Regulations, as set forth below.
End Amendment Part Start PartPART 17—[AMENDED]
End Part Start Amendment Part1. The authority citation for part 17 continues to read as follows:
End Amendment Part Start Amendment Part2. In § 17.11(h), the table entry for “Bat, Mariana fruit” under MAMMALS is revised to read as follows:
End Amendment PartStart SignatureEndangered and threatened wildlife.* * * * *(h) * * *
Species Historic range Vertebrate population where endangered or threatened Status When listed Critical habitat Special rules Common name Scientific name * * * * * * * Mammals * * * * * * * Fruit Bat, Mariana (=fanihi, Mariana flying fox) Pteropus mariannus mariannus Western Pacific Ocean—U.S.A. (GU, MP) Entire T 156 Guam 17.95(a) NA End Signature End Supplemental InformationDated: December 30, 2004.
Steve Williams,
Director, Fish and Wildlife Service.
BILLING CODE 4310-55-P
BILLING CODE 4310-55-C
[FR Doc. 05-240 Filed 1-5-05; 8:45 am]
BILLING CODE 4310-55-P
Document Information
- Effective Date:
- 2/7/2005
- Published:
- 01/06/2005
- Department:
- Fish and Wildlife Service
- Entry Type:
- Rule
- Action:
- Final rule.
- Document Number:
- 05-240
- Dates:
- This final rule is effective February 7, 2005.
- Pages:
- 1190-1210 (21 pages)
- RINs:
- 1018-AH55: Endangered and Threatened Wildlife; Reclassification from Endangered to Threatened Status for Marianna Fruit Bat (Guam), and Threatened Status for Marianna Fruit Bat (CNMI)
- RIN Links:
- https://www.federalregister.gov/regulations/1018-AH55/endangered-and-threatened-wildlife-reclassification-from-endangered-to-threatened-status-for-mariann
- Topics:
- Endangered and threatened species, Exports, Imports, Reporting and recordkeeping requirements, Transportation
- PDF File:
- 05-240.pdf
- CFR: (1)
- 50 CFR 17.11