-
Start Preamble
AGENCY:
National Institutes of Health, Public Health Service, HHS.
ACTION:
Notice.
SUMMARY:
The inventions listed below are owned by an agency of the U.S. Government and are available for licensing in the U.S. in accordance with 35 U.S.C. 207 to achieve expeditious commercialization of results of federally-funded research and development. Foreign patent applications are filed on selected inventions to extend market coverage for companies and may also be available for licensing.
ADDRESSES:
Licensing information and copies of the U.S. patent applications listed below may be obtained by writing to the indicated licensing contact at the Office of Technology Transfer, National Institutes of Health, 6011 Executive Boulevard, Suite 325, Rockville, Maryland 20852-3804; telephone: 301/496-7057; fax: 301/402-0220. A signed Confidential Disclosure Agreement will be required to receive copies of the patent applications.
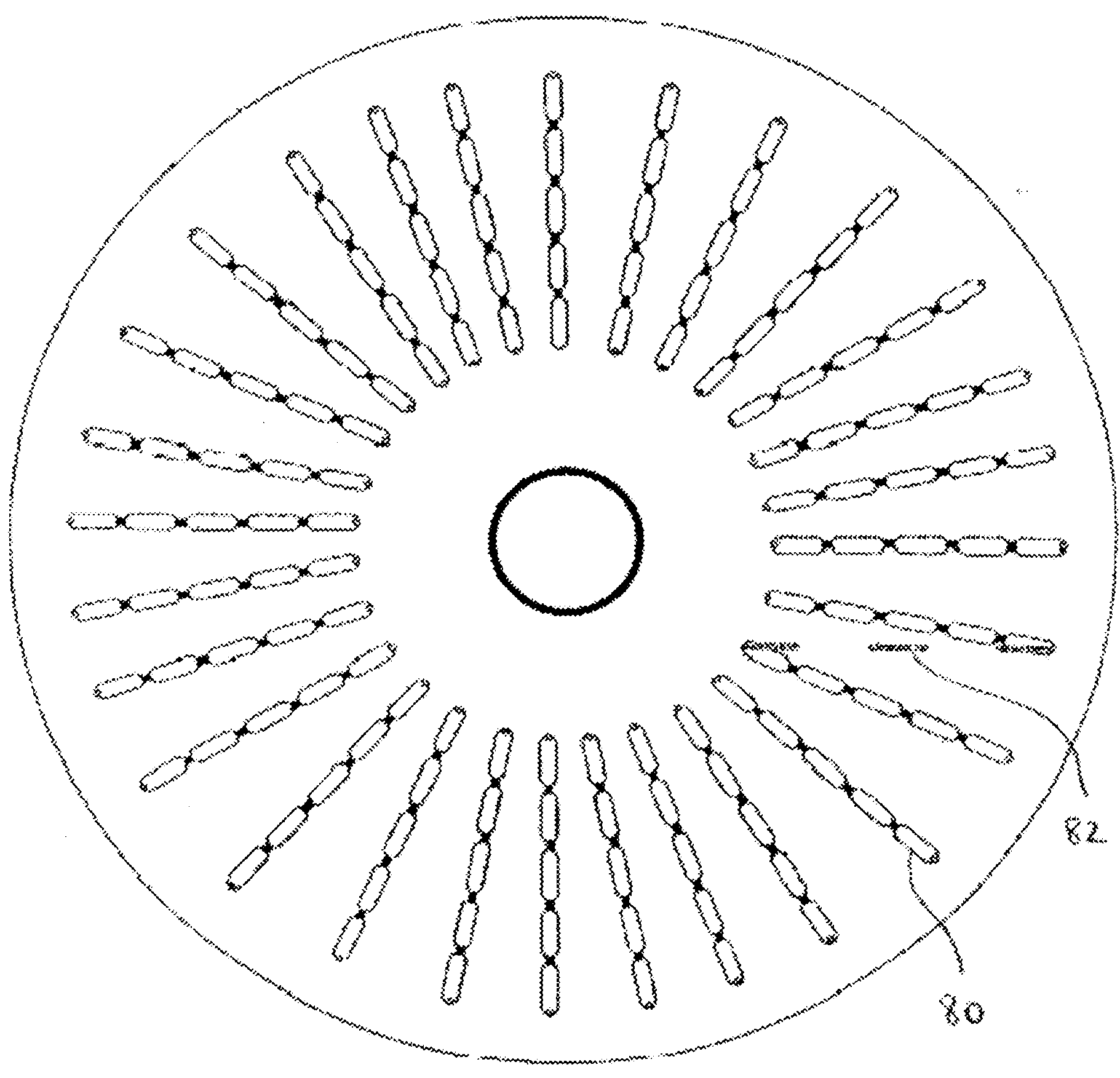
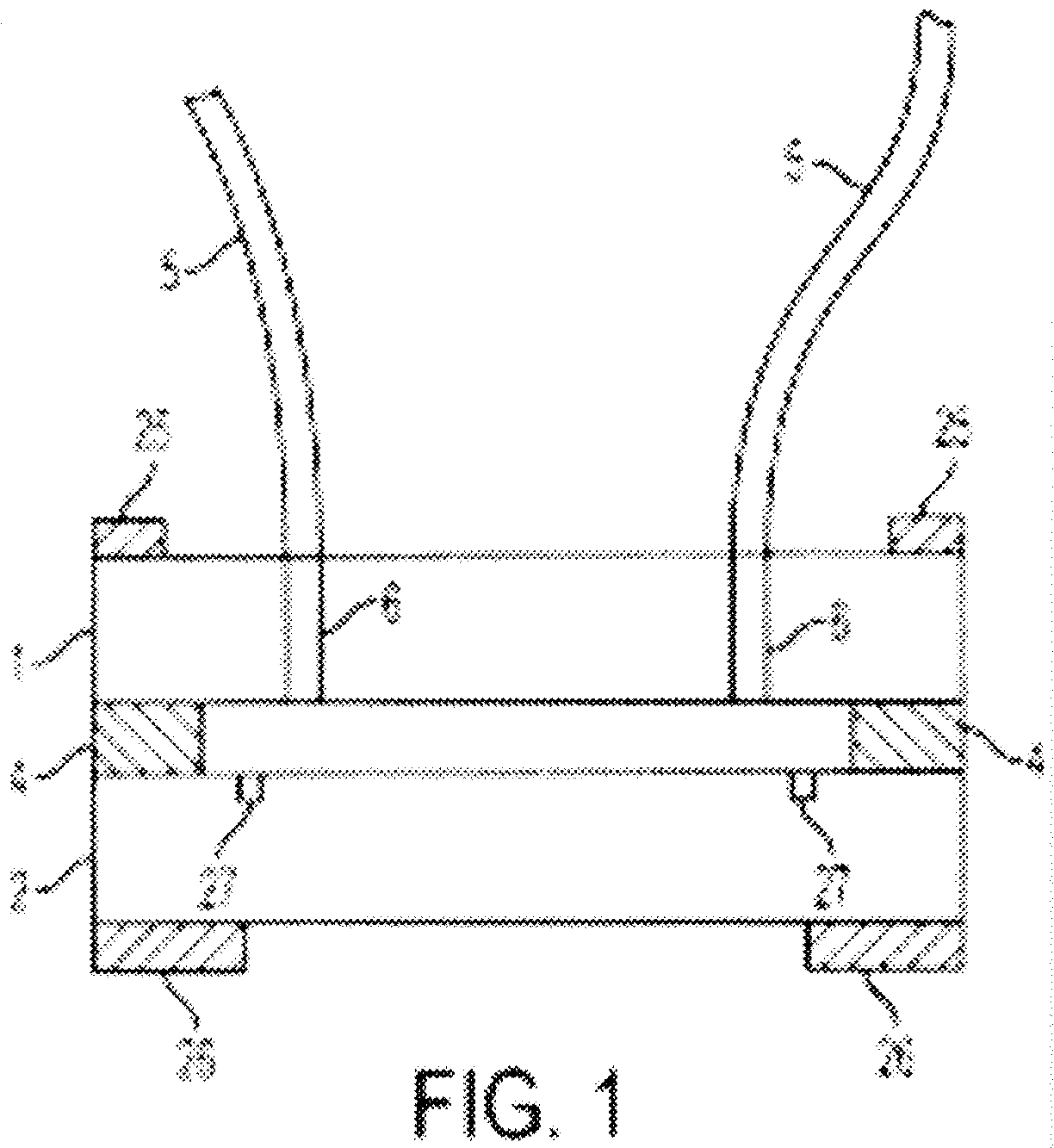
Chromatography Apparatus and Method
Yoichiro Ito (NHLBI)
U.S. Provisional Application Filed 24 Aug 2004 (DHHS Reference No. E-277-2004/0-US-01)
Licensing Contact: Michael Shmilovich; 301/435-5019; shmilovm@mail.nih.gov.
Available for licensing for industrial scale-up production and commercial distribution is an improved countercurrent chromatography apparatus comprising a disk having a series of interconnected and elongated compartments coupled by ducts that form a portion of a groove in a surface of the disk. At least some of the elongated compartments have an aspect ratio of at least greater than two and a width greater than twice the width of the connecting ducts and a length of about 10 to 20 times the length of the connecting ducts. This apparatus may also be used for a large-scale industrial separation by coaxially rotating in centrifugal or gravitational fields.
HIV-1 Infection Detection Assay for Seroconverted HIV-1 Vaccine Recipients
Hana Golding, Surender Khurana (FDA/CBER)
U.S. Provisional Application Filed 08 Sep 2004 (DHHS Reference No. E-259-2004/0-US-01)
Licensing Contact: Michael Shmilovich; 301/435-5019; shmilovm@mail.nih.gov.
Available for licensing and commercial distribution is an assay method and kit having diagnostic peptide fragments derived from human immunodeficiency virus-1 (HIV-1). The new serology assay includes HIV-1 peptide fragments epitopes that map to HIV-1 GAG-p6, and gp41 genes. These epitopes are broadly reactive with early sera from HIV infected individuals, do not illicit protective antibodies, do not illicit immunologic cytotoxicity and are readily removable from current and future HIV-1 candidates. The assay is advantageous in detecting HIV-1 early breakthrough infections in seroconverted vaccine recipients while being able to distinguish between individuals with bonafide breakthrough infections versus non-HIV infected vaccine recipients presenting only vaccine borne antibodies. For example, 90% of vaccine recipients receiving a Canarypox construct expressing a plurality of HIV antigens (Env, Gag, Pol, HIV Protease, Nef) followed by an envelope protein boost, scored positive in FDA licensed enzyme immunoassay, rapid test, and Western blot (Marta-Louise Ackers et al., J Infect Dis. 187:879 (2003)). Such seroconversion has a negative impact on phase III efficacy trials of prophylactic HIV vaccines that require early detection of breakthrough infections and also exclude non-HIV infected vaccine recipients from the pool of potential blood donors.
Flow-Through, Thermal-Expansion-Compensated Microcells for Analytical Transmission Infrared and Other Light Spectroscopies
Edward Mertz (NICHD), James Sullivan (ORS) Start Printed Page 59262
U.S. Patent Application No. 10/926,405 Filed 26 Aug 2004 (DHHS Reference No. E-096-2004/0-US-01)
Licensing Contact: Michael Shmilovich; 301/435-5019; shmilovm@mail.nih.gov.
Available for licensing and commercial distribution are optical cells that are spectroscopically, thermally and mechanically stable and can be used for spectroscopic measurement in transmission, reflection, transmission-reflection, emission, or scattering modes without modification of standard spectrometers. The cell handles liquid samples and biological or solid samples equilibrated with bathing fluid which does not interfere with the light beam, allows liquid sample or bathing fluid to be exchanged without cell reassembly, requires only a small amount of sample (down to 0.1μl), allows for different sample gaps (0.2-1000μm) to be easily and inexpensively set, and allows spectral measurements to be taken over wavelengths ranging at least from the mid-infrared to the vacuum ultraviolet. The inventive cell and methods allows sensitive and reproducible monitoring spectra and their changes (down to at least 10−4 absorbance units) caused by changes in temperature or in composition of bathing fluid or by fast kinetic processes.
This research is described, in part, in Mertz E.L., Leikin S. “Interactions of Inorganic Phosphate and Sulfate Anions with Collagen”, Biochemistry, in press.
Device for Sequential Protein Transfer From a Gel
Jozsef Antal, Zsuzsanna Buzas, Andreas Chrambach (NICHD)
DHHS Reference No. E-346-2003/0-US-01 filed 09 July 2004
Licensing Contact: Michael Shmilovich; 301/435-5019; shmilovm@mail.nih.gov.
Available for licensing and commercialization is a device for sequentially eluting proteins and peptides. The device comprises a separation medium having an outlet, and a collector having a first receptacle and second receptacle that can be sequentially brought into contact with the outlet of the separation medium by translating (rotating) the first receptacle and the second receptacle in relation to the outlet of the separation medium. The invention is adaptable to capillary electrophoresis as well. Multiple sequential protein transfer from SDS-PAGE gel to a mass spectrometer is made possible. Separated protein bands sequentially electrophorese into low melting agarose plugs distributed along the surface of a plastic drum. The effective electroelution of a protein from a gel band to an agarose filled slot. The drum is rotated to receive each band individually. Migrating SDS linearized proteins are electrophoresed into the receptacle slot drum. The drum is rolled until each protein of interest is separated. Agarose plugs are lifted from the drum slots; enzymatically dissolved, and loaded directly onto a MALDI spectrometer. Between two agarose layers, gel free collection chambers can be formed inside the drum providing solution phase fraction collection.
This research is described in: Buzas Z, Antal J, Gilligan JJ, Backlund PS, Yergey AL, Chrambach A. An electroelution apparatus for sequential transfer of sodium dodecyl sulfate-proteins into agarose and mass spectrometric identification of Li-Na-dodecyl sulfate-proteins from solubilized agarose. Electrophoresis. 2004 Apr;25(7-8):966-9.
Simultaneous HDL/LDL/Total Lipoprotein Single Tube Homogeneous Assay
Alan T. Remaley, Maureen Sampson, Gyorgy Csako (CC)
DHHS Reference No. E-090-1999: U.S. Patent App. 09/980,751 Filed 01 Nov 2001; European Patent App. Ser. No. 00939404.0 Filed 26 May 2000; Canadian Patent. App. 2375210 Filed 26 May 2000; Australian Pat. App. 54493/00 filed 26 May 2000; Japanese Patent App. 2001-500866 filed 26 May 2000
Licensing Contact: Michael Shmilovich; 301/435-5019; shmilovm@mail.nih.gov.
Available for licensing is an invention in which a single tube assay is used for determining high-density lipoprotein HDL-cholesterol (HDL-C), low density lipoprotein (LDL-C) and total cholesterol (total-C), from a single serum sample. This assay is an efficient tool for use in determining patient risk factors for heart disease. Previously, multiple costly tests were performed in order to determine low-density lipoprotein LDL-C and HDL-C by measuring total-C, total triglyceride, and HDL-C. That method of testing had limitations and was complex. Using this methodology, the homogeneous assay for HDL-C does not require physically separating HDL. The new assay developed is efficient, less costly, and compares favorably to current assays for HDL-C, total cholesterol, and triglyceride. This technology may also be used to simplify the procedure for the point of care testing of hyperlipidemia.
Start SignatureEnd Signature End PreambleDated: September 22, 2004.
Steven M. Ferguson,
Director, Division of Technology Development and Transfer, Office of Technology Transfer, National Institutes of Health.
[FR Doc. 04-22151 Filed 10-1-04; 8:45 am]
BILLING CODE 4140-01-P
Document Information
- Published:
- 10/04/2004
- Department:
- National Institutes of Health
- Entry Type:
- Notice
- Action:
- Notice.
- Document Number:
- 04-22151
- Pages:
- 59261-59262 (2 pages)
- PDF File:
- 04-22151.pdf