2013-03480. Medicare and Medicaid Programs; Quarterly Listing of Program Issuances-October Through December 2012
-
Start Preamble
AGENCY:
Centers for Medicare & Medicaid Services (CMS), HHS.
ACTION:
Notice.
SUMMARY:
This quarterly notice lists CMS manual instructions, substantive and interpretive regulations, and other Federal Register notices that were published from October through December 2012, relating to the Medicare and Medicaid programs and other programs administered by CMS.
Start Further InfoFOR FURTHER INFORMATION CONTACT:
It is possible that an interested party may need specific information and not be able to determine from the listed information whether the issuance or regulation would fulfill that need. Start Printed Page 11190Consequently, we are providing contact persons to answer general questions concerning each of the addenda published in this notice.
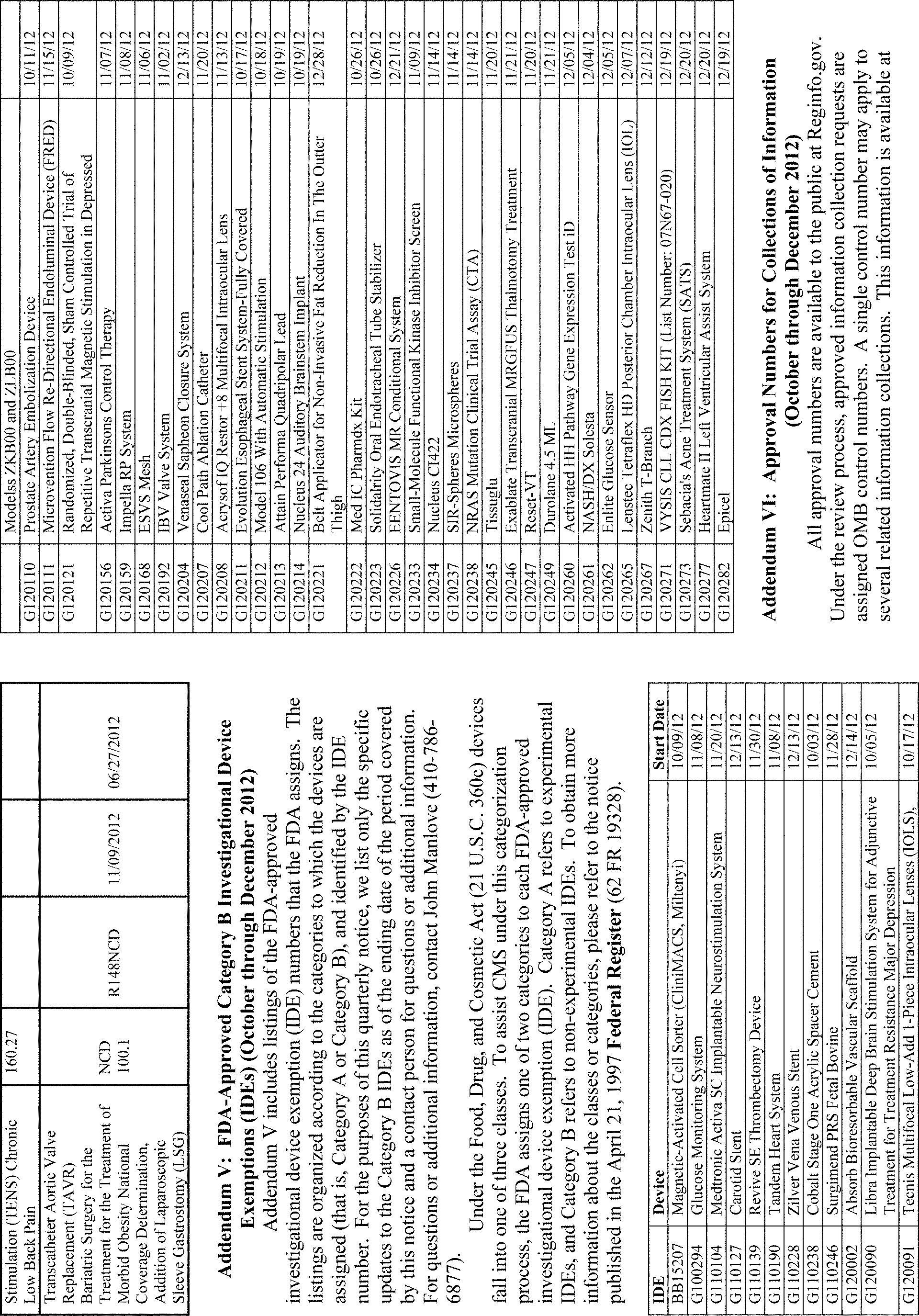
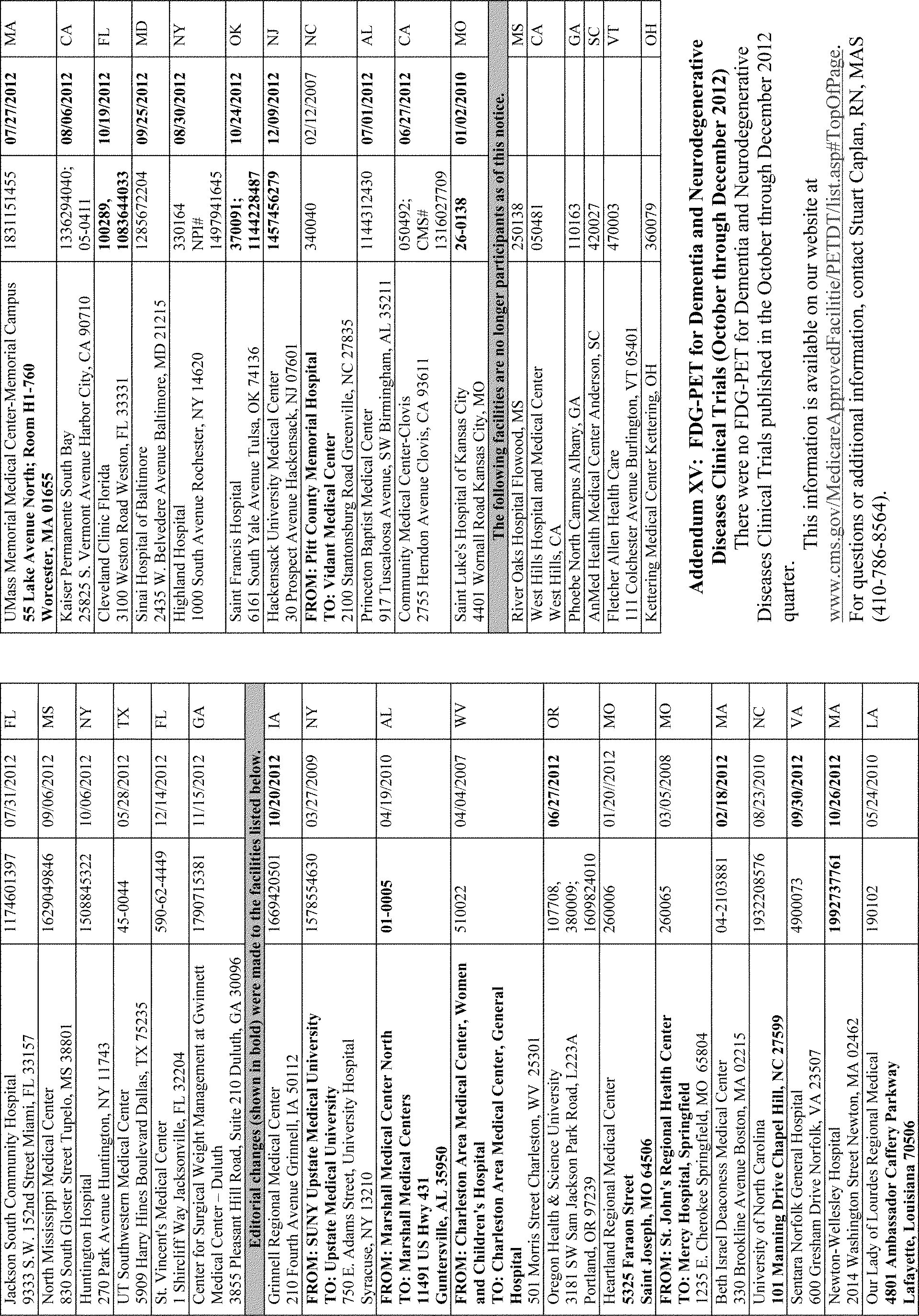
Addenda Contact Phone No. I CMS Manual Instructions Ismael Torres (410) 786-1864 II Regulation Documents Published in the Federal Register Terri Plumb (410) 786-4481 III CMS Rulings Tiffany Lafferty (410) 786-7548 IV Medicare National Coverage Determinations Wanda Belle (410) 786-7491 V FDA-Approved Category B IDEs John Manlove (410) 786-6877 VI Collections of Information Mitch Bryman (410) 786-5258 VII Medicare-Approved Carotid Stent Facilities Sarah J. McClain (410) 786-2294 VIII American College of Cardiology-National Cardiovascular Data Registry Sites JoAnna Baldwin, MS (410) 786-7205 IX Medicare's Active Coverage-Related Guidance Documents Lori Ashby (410) 786-6322 X One-time Notices Regarding National Coverage Provisions Lori Ashby (410) 786-6322 XI National Oncologic Positron Emission Tomography Registry Sites Stuart Caplan, RN, MAS (410) 786-8564 XII Medicare-Approved Ventricular Assist Device (Destination Therapy) Facilities JoAnna Baldwin, MS (410) 786-7205 XIII Medicare-Approved Lung Volume Reduction Surgery Facilities JoAnna Baldwin, MS (410) 786-7205 XIV Medicare-Approved Bariatric Surgery Facilities Kate Tillman, RN, MAS (410) 786-9252 XV Fluorodeoxyglucose Positron Emission Tomography for Dementia Trials Stuart Caplan, RN, MAS (410) 786-8564 All Other Information Annette Brewer (410) 786-6580 I. Background
Among other things, the Centers for Medicare & Medicaid Services (CMS) is responsible for administering the Medicare and Medicaid programs and coordination and oversight of private health insurance. Administration and oversight of these programs involves the following: (1) Furnishing information to Medicare and Medicaid beneficiaries, health care providers, and the public; and (2) maintaining effective communications with CMS regional offices, state governments, state Medicaid agencies, state survey agencies, various providers of health care, all Medicare contractors that process claims and pay bills, National Association of Insurance Commissioners (NAIC), health insurers, and other stakeholders. To implement the various statutes on which the programs are based, we issue regulations under the authority granted to the Secretary of the Department of Health and Human Services under sections 1102, 1871, 1902, and related provisions of the Social Security Act (the Act) and Public Health Service Act. We also issue various manuals, memoranda, and statements necessary to administer and oversee the programs efficiently.
Section 1871(c) of the Act requires that we publish a list of all Medicare manual instructions, interpretive rules, statements of policy, and guidelines of general applicability not issued as regulations at least every 3 months in the Federal Register.
II. Revised Format for the Quarterly Issuance Notices
While we are publishing the quarterly notice required by section 1871(c) of the Act, we will no longer republish duplicative information that is available to the public elsewhere. We believe this approach is in alignment with CMS' commitment to the general principles of the President's Executive Order 13563 released January 2011 entitled “Improving Regulation and Regulatory Review,” which promotes modifying and streamlining an agency's regulatory program to be more effective in achieving regulatory objectives. Section 6 of Executive Order 13563 requires agencies to identify regulations that may be “outmoded, ineffective, insufficient, or excessively burdensome, and to modify, streamline, expand or repeal them in accordance with what has been learned.” This approach is also in alignment with the President's Open Government and Transparency Initiative that establishes a system of transparency, public participation, and collaboration.
Therefore, this quarterly notice provides only the specific updates that have occurred in the 3-month period along with a hyperlink to the full listing that is available on the CMS Web site or the appropriate data registries that are used as our resources. This information is the most current up-to-date information and will be available earlier than we publish our quarterly notice. We believe the Web site list provides more timely access for beneficiaries, providers, and suppliers. We also believe the Web site offers a more convenient tool for the public to find the full list of qualified providers for these specific services and offers more flexibility and “real time” accessibility. In addition, many of the Web sites have listservs; that is, the public can subscribe and receive immediate notification of any updates to the Web site. These listservs avoid the need to check the Web site, as notification of updates is automatic and sent to the subscriber as they occur. If assessing a Web site proves to be difficult, the contact person listed can provide information.
III. How To Use the Notice
This notice is organized into 15 addenda so that a reader may access the subjects published during the quarter covered by the notice to determine whether any are of particular interest. We expect this notice to be used in concert with previously published notices. Those unfamiliar with a description of our Medicare manuals should view the manuals at http://www.cms.gov/manuals.
Start SignatureEnd Signature Start Printed Page 11191 Start Printed Page 11192 Start Printed Page 11193 Start Printed Page 11194 Start Printed Page 11195 Start Printed Page 11196 Start Printed Page 11197 Start Printed Page 11198 Start Printed Page 11199 Start Printed Page 11200 Start Printed Page 11201 End Further Info End PreambleDated: February 8, 2013.
Kathleen Cantwell,
Director, Office of Strategic Operations and Regulatory Affairs.
[FR Doc. 2013-03480 Filed 2-14-13; 8:45 am]
BILLING CODE 4120-01-P
Document Information
- Comments Received:
- 0 Comments
- Published:
- 02/15/2013
- Department:
- Centers for Medicare & Medicaid Services
- Entry Type:
- Notice
- Action:
- Notice.
- Document Number:
- 2013-03480
- Pages:
- 11189-11201 (13 pages)
- Docket Numbers:
- CMS-9076-N
- PDF File:
- 2013-03480.pdf