E9-6148. Medicare and Medicaid Programs; Quarterly Listing of Program Issuances-October Through December 2008
-
Start Preamble
Start Printed Page 13516
AGENCY:
Centers for Medicare & Medicaid Services (CMS), HHS.
ACTION:
Notice.
SUMMARY:
This notice lists CMS manual instructions, substantive and interpretive regulations, and other Federal Register notices that were published from October 2008 through December 2008, relating to the Medicare and Medicaid programs. This notice provides information on national coverage determinations (NCDs) affecting specific medical and health care services under Medicare. Additionally, this notice identifies certain devices with investigational device exemption (IDE) numbers approved by the Food and Drug Administration (FDA) that potentially may be covered under Medicare. This notice also includes listings of all approval numbers from the Office of Management and Budget for collections of information in CMS regulations and a list of Medicare-approved carotid stent facilities. Included in this notice is a list of the American College of Cardiology's National Cardiovascular Data registry sites, active CMS coverage-related guidance documents, and special one-time notices regarding national coverage provisions. Also included in this notice is a list of National Oncologic Positron Emissions Tomography Registry sites, a list of Medicare-approved ventricular assist device (destination therapy) facilities, a list of Medicare-approved lung volume reduction surgery facilities, a list of Medicare-approved clinical trials for fluorodeoxyglucose positron emissions tomography for dementia, and a list of Medicare-approved bariatric surgery facilities.
Section 1871(c) of the Social Security Act requires that we publish a list of Medicare issuances in the Federal Register at least every 3 months. Although we are not mandated to do so by statute, for the sake of completeness of the listing, and to foster more open and transparent collaboration efforts, we are also including all Medicaid issuances and Medicare and Medicaid substantive and interpretive regulations (proposed and final) published during this 3-month time frame.
Start Further InfoFOR FURTHER INFORMATION CONTACT:
It is possible that an interested party may need specific information and not be able to determine from the listed information whether the issuance or regulation would fulfill that need. Consequently, we are providing contact persons to answer general questions concerning these items. Copies are not available through the contact persons. (See Section III of this notice for how to obtain listed material.)
Questions concerning CMS manual instructions in Addendum III may be addressed to Ismael Torres, Office of Strategic Operations and Regulatory Affairs, Centers for Medicare & Medicaid Services, C4-26-05, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-1864.
Questions concerning regulation documents published in the Federal Register in Addendum IV may be addressed to Gwendolyn Johnson, Office of Strategic Operations and Regulatory Affairs, Centers for Medicare & Medicaid Services, C4-14-03, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-6954.
Questions concerning Medicare NCDs in Addendum V may be addressed to Patricia Brocato-Simons, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-0261.
Questions concerning FDA-approved Category B IDE numbers listed in Addendum VI may be addressed to John Manlove, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-13-04, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-6877.
Questions concerning approval numbers for collections of information in Addendum VII may be addressed to Melissa Musotto, Office of Strategic Operations and Regulatory Affairs, Regulations Development and Issuances Group, Centers for Medicare & Medicaid Services, C5-14-03, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-6962.
Questions concerning Medicare-approved carotid stent facilities in Addendum VIII may be addressed to Sarah J. McClain, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-2994.
Questions concerning Medicare's recognition of the American College of Cardiology—National Cardiovascular Data Registry sites in Addendum IX may be addressed to JoAnna Baldwin, MS, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-7205.
Questions concerning Medicare's active coverage-related guidance documents in Addendum X may be addressed to Beverly Lofton, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-7136.
Questions concerning one-time notices regarding national coverage provisions in Addendum XI may be addressed to Beverly Lofton, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-7136.
Questions concerning National Oncologic Positron Emission Tomography Registry sites in Addendum XII may be addressed to Stuart Caplan, RN, MAS, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-8564.
Questions concerning Medicare-approved ventricular assist device (destination therapy) facilities in Addendum XIII may be addressed to JoAnna Baldwin, MS, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-7205.
Questions concerning Medicare-approved lung volume reduction surgery facilities listed in Addendum XIV may be addressed to JoAnna Baldwin, MS, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-7205.
Questions concerning Medicare-approved bariatric surgery facilities listed in Addendum XV may be addressed to Kate Tillman, RN, MA, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-9252.
Questions concerning fluorodeoxyglucose positron emission Start Printed Page 13517tomography for dementia trials listed in Addendum XVI may be addressed to Stuart Caplan, RN, MAS, Office of Clinical Standards and Quality, Centers for Medicare & Medicaid Services, C1-09-06, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-8564.
Questions concerning all other information may be addressed to Gwendolyn Johnson, Office of Strategic Operations and Regulatory Affairs, Regulations Development Group, Centers for Medicare & Medicaid Services, C5-14-03, 7500 Security Boulevard, Baltimore, MD 21244-1850, or you can call (410) 786-6954.
End Further Info End Preamble Start Supplemental InformationSUPPLEMENTARY INFORMATION:
I. Program Issuances
The Centers for Medicare & Medicaid Services (CMS) is responsible for administering the Medicare and Medicaid programs. These programs pay for health care and related services for 39 million Medicare beneficiaries and 35 million Medicaid recipients. Administration of the two programs involves (1) furnishing information to Medicare beneficiaries and Medicaid recipients, health care providers, and the public and (2) maintaining effective communications with regional offices, State governments, State Medicaid agencies, State survey agencies, various providers of health care, all Medicare contractors that process claims and pay bills, and others. To implement the various statutes on which the programs are based, we issue regulations under the authority granted to the Secretary of the Department of Health and Human Services under sections 1102, 1871, 1902, and related provisions of the Social Security Act (the Act). We also issue various manuals, memoranda, and statements necessary to administer the programs efficiently.
Section 1871(c)(1) of the Act requires that we publish a list of all Medicare manual instructions, interpretive rules, statements of policy, and guidelines of general applicability not issued as regulations at least every 3 months in the Federal Register. We published our first notice June 9, 1988 (53 FR 21730). Although we are not mandated to do so by statute, for the sake of completeness of the listing of operational and policy statements, and to foster more open and transparent collaboration, we are continuing our practice of including Medicare substantive and interpretive regulations (proposed and final) published during the respective 3-month time frame.
II. How To Use the Addenda
This notice is organized so that a reader may review the subjects of manual issuances, memoranda, substantive and interpretive regulations, NCDs, and FDA-approved IDEs published during the subject quarter to determine whether any are of particular interest. We expect this notice to be used in concert with previously published notices. Those unfamiliar with a description of our Medicare manuals may wish to review Table I of our first three notices (53 FR 21730, 53 FR 36891, and 53 FR 50577) published in 1988, and the notice published March 31, 1993 (58 FR 16837). Those desiring information on the Medicare NCD Manual (NCDM, formerly the Medicare Coverage Issues Manual (CIM)) may wish to review the August 21, 1989, publication (54 FR 34555). Those interested in the revised process used in making NCDs under the Medicare program may review the September 26, 2003, publication (68 FR 55634).
To aid the reader, we have organized and divided this current listing into 11 addenda:
- Addendum I lists the publication dates of the most recent quarterly listings of program issuances.
- Addendum II identifies previous Federal Register documents that contain a description of all previously published CMS Medicare and Medicaid manuals and memoranda.
- Addendum III lists a unique CMS transmittal number for each instruction in our manuals or Program Memoranda and its subject matter. A transmittal may consist of a single or multiple instruction(s). Often, it is necessary to use information in a transmittal in conjunction with information currently in the manuals.
- Addendum IV lists all substantive and interpretive Medicare and Medicaid regulations and general notices published in the Federal Register during the quarter covered by this notice. For each item, we list the—
○ Date published;
○ Federal Register citation;
○ Parts of the Code of Federal Regulations (CFR) that have changed (if applicable);
○ Agency file code number; and
○ Title of the regulation.
- Addendum V includes completed NCDs, or reconsiderations of completed NCDs, from the quarter covered by this notice. Completed decisions are identified by the section of the NCDM in which the decision appears, the title, the date the publication was issued, and the effective date of the decision.
- Addendum VI includes listings of the FDA-approved IDE categorizations, using the IDE numbers the FDA assigns. The listings are organized according to the categories to which the device numbers are assigned (that is, Category A or Category B), and identified by the IDE number.
- Addendum VII includes listings of all approval numbers from the Office of Management and Budget (OMB) for collections of information in CMS regulations in title 42; title 45, subchapter C; and title 20 of the CFR.
- Addendum VIII includes listings of Medicare-approved carotid stent facilities. All facilities listed meet CMS standards for performing carotid artery stenting for high risk patients.
- Addendum IX includes a list of the American College of Cardiology's National Cardiovascular Data registry sites. We cover implantable cardioverter defibrillators (ICDs) for certain indications, as long as information about the procedures is reported to a central registry.
- Addendum X includes a list of active CMS guidance documents. As required by section 731 of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) (Pub. L. 108-173, enacted on December 8, 2003), we will begin listing the current versions of our guidance documents in each quarterly listings notice.
- Addendum XI includes a list of special one-time notices regarding national coverage provisions. We are publishing a list of issues that require public notification, such as a particular clinical trial or research study that qualifies for Medicare coverage.
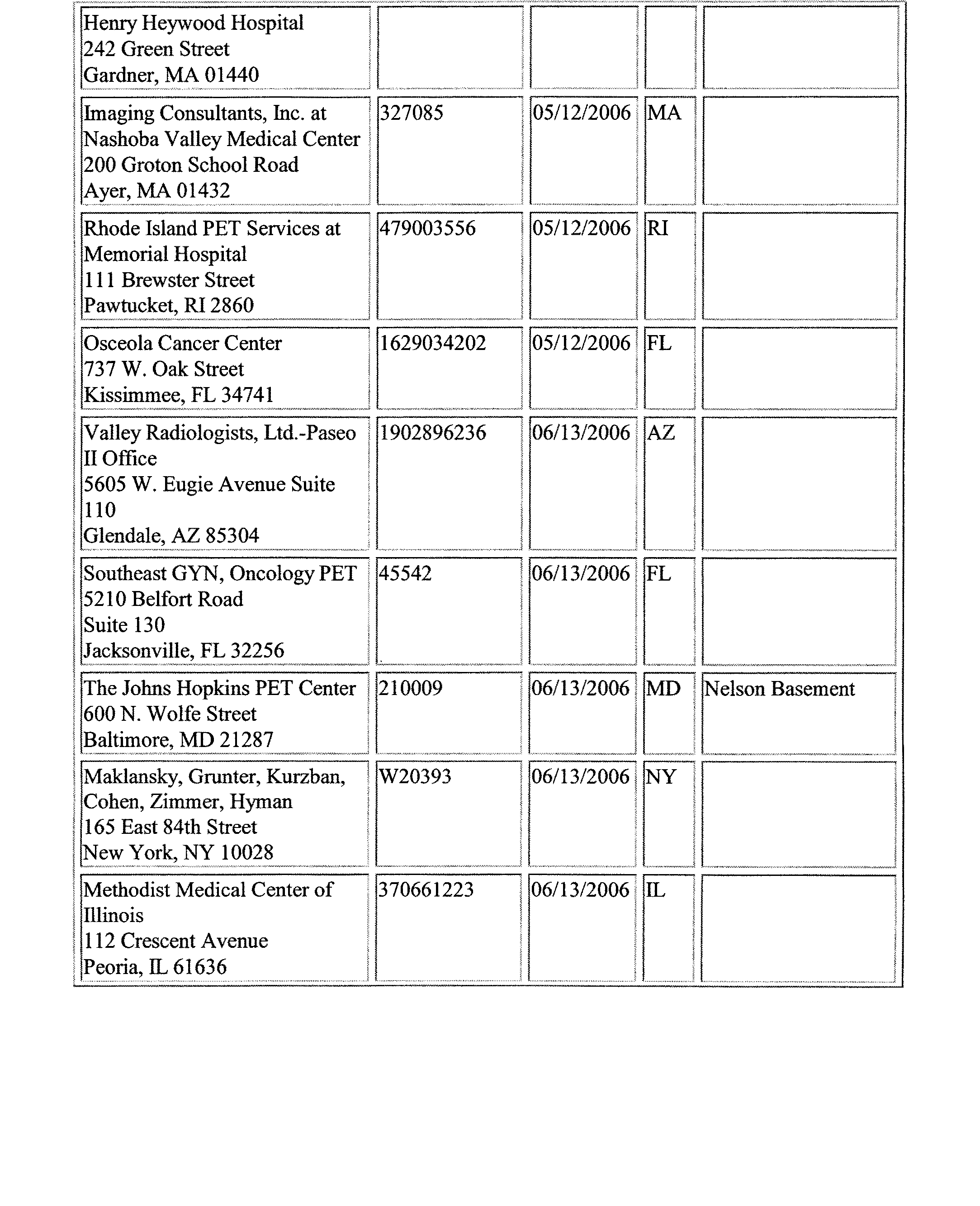
- Addendum XII includes a listing of National Oncologic Positron Emission Tomography Registry (NOPR) sites. We cover positron emission tomography (PET) scans for particular oncologic indications when they are performed in a facility that participates in the NOPR.
- Addendum XIII includes a listing of Medicare-approved facitilites that receive coverage for ventricular assist devices used as destination therapy. All facilities were required to meet our standards in order to receive coverage for ventricular assist devices implanted as destination therapy.
- Addendum XIV includes a listing of Medicare-approved facilities that are eligible to receive coverage for lung volume reduction surgery. Until May 17, 2007, facilities that participated in the National Emphysema Treatment Trial are also eligible to receive coverage.
- Addendum XV includes a listing of Medicare-approved facilities that meet minimum standards for facilities modeled in part on professional society statements on competency. All facilities Start Printed Page 13518must meet our standards in order to receive coverage for bariatric surgery procedures.
- Addendum XVI includes a listing of Medicare-approved clinical trials for fluorodeoxyglucose positron emission tomography (FDG-PET) for dementia and neurodegenerative diseases.
III. How To Obtain Listed Material
A. Manuals
Those wishing to subscribe to program manuals should contact either the Government Printing Office (GPO) or the National Technical Information Service (NTIS) at the following addresses: Superintendent of Documents, Government Printing Office, ATTN: New Orders, P.O. Box 371954, Pittsburgh, PA 15250-7954, Telephone (202) 512-1800, Fax number (202) 512-2250 (for credit card orders); or National Technical Information Service, Department of Commerce, 5825 Port Royal Road, Springfield, VA 22161, Telephone (703) 487-4630.
In addition, individual manual transmittals and Program Memoranda listed in this notice can be purchased from NTIS. Interested parties should identify the transmittal(s) they want. GPO or NTIS can give complete details on how to obtain the publications they sell. Additionally, most manuals are available at the following Internet address: http://cms.hhs.gov/manuals/default.asp.
B. Regulations and Notices
Regulations and notices are published in the daily Federal Register. Interested individuals may purchase individual copies or subscribe to the Federal Register by contacting the GPO at the address given above. When ordering individual copies, it is necessary to cite either the date of publication or the volume number and page number.
The Federal Register is also available on 24x microfiche and as an online database through GPO Access. The online database is updated by 6 a.m. each day the Federal Register is published. The database includes both text and graphics from Volume 59, Number 1 (January 2, 1994) forward. Free public access is available on a Wide Area Information Server (WAIS) through the Internet and via asynchronous dial-in. Internet users can access the database by using the World Wide Web; the Superintendent of Documents home page address is http://www.gpoaccess.gov/fr/index.html, by using local WAIS client software, or by telnet to swais.gpoaccess.gov, then log in as guest (no password required). Dial-in users should use communications software and modem to call (202) 512-1661; type swais, then log in as guest (no password required).
C. Rulings
We publish rulings on an infrequent basis. CMS Rulings are decisions of the Administrator that serve as precedent final opinions and orders and statements of policy and interpretation. They provide clarification and interpretation of complex or ambiguous provisions of the law or regulations relating to Medicare, Medicaid, Utilization and Quality Control Peer Review, private health insurance, and related matters. Interested individuals can obtain copies from the nearest CMS Regional Office or review them at the nearest regional depository library. We have, on occasion, published rulings in the Federal Register. Rulings, beginning with those released in 1995, are available online, through the CMS Home Page. The Internet address is http://cms.hhs.gov/rulings.
D. CMS' Compact Disk-Read Only Memory (CD-ROM)
Our laws, regulations, and manuals are also available on CD-ROM and may be purchased from GPO or NTIS on a subscription or single copy basis. The Superintendent of Documents list ID is HCLRM, and the stock number is 717-139-00000-3. The following material is on the CD-ROM disk:
- Titles XI, XVIII, and XIX of the Act.
- CMS-related regulations.
- CMS manuals and monthly revisions.
- CMS program memoranda.
The titles of the Compilation of the Social Security Laws are current as of January 1, 2005. (Updated titles of the Social Security Laws are available on the Internet at http://www.ssa.gov/OP_Home/ssact/comp-toc.htm.) The remaining portions of CD-ROM are updated on a monthly basis.
Because of complaints about the unreadability of the Appendices (Interpretive Guidelines) in the State Operations Manual (SOM), as of March 1995, we deleted these appendices from CD-ROM. We intend to re-visit this issue in the near future and, with the aid of newer technology, we may again be able to include the appendices on CD-ROM.
Any cost report forms incorporated in the manuals are included on the CD-ROM disk as LOTUS files. LOTUS software is needed to view the reports once the files have been copied to a personal computer disk.
IV. How To Review Listed Material
Transmittals or Program Memoranda can be reviewed at a local Federal Depository Library (FDL). Under the FDL program, government publications are sent to approximately 1,400 designated libraries throughout the United States. Some FDLs may have arrangements to transfer material to a local library not designated as an FDL. Contact any library to locate the nearest FDL.
In addition, individuals may contact regional depository libraries that receive and retain at least one copy of most Federal Government publications, either in printed or microfilm form, for use by the general public. These libraries provide reference services and interlibrary loans; however, they are not sales outlets. Individuals may obtain information about the location of the nearest regional depository library from any library.
For each CMS publication listed in Addendum III, CMS publication and transmittal numbers are shown. To help FDLs locate the materials, use the CMS publication and transmittal numbers. For example, to find the Medicare Benefit Policy publication titled “Continuous Positive Airway Pressure Therapy for Obstructive Sleep Apnea,” use CMS-Pub. 100-03, Transmittal No. 96.
(Catalog of Federal Domestic Assistance Program No. 93.773, Medicare—Hospital Insurance, Program No. 93.774, Medicare—Supplementary Medical Insurance Program, and Program No. 93.714, Medical Assistance Program)
Start SignatureEnd Signature Start Printed Page 13519 Start Printed Page 13520 Start Printed Page 13521 Start Printed Page 13522 Start Printed Page 13523 Start Printed Page 13524 Start Printed Page 13525 Start Printed Page 13526 Start Printed Page 13527 Start Printed Page 13528 Start Printed Page 13529 Start Printed Page 13530 Start Printed Page 13531 Start Printed Page 13532 Start Printed Page 13533 Start Printed Page 13534 Start Printed Page 13535 Start Printed Page 13536 Start Printed Page 13537 Start Printed Page 13538 Start Printed Page 13539 Start Printed Page 13540 Start Printed Page 13541 Start Printed Page 13542 Start Printed Page 13543 Start Printed Page 13544 Start Printed Page 13545 Start Printed Page 13546 Start Printed Page 13547 Start Printed Page 13548 Start Printed Page 13549 Start Printed Page 13550 Start Printed Page 13551 Start Printed Page 13552 Start Printed Page 13553 Start Printed Page 13554 Start Printed Page 13555 Start Printed Page 13556 Start Printed Page 13557 Start Printed Page 13558 Start Printed Page 13559 Start Printed Page 13560 Start Printed Page 13561 Start Printed Page 13562 Start Printed Page 13563 Start Printed Page 13564 Start Printed Page 13565 Start Printed Page 13566 Start Printed Page 13567 Start Printed Page 13568 Start Printed Page 13569 Start Printed Page 13570 Start Printed Page 13571 Start Printed Page 13572 Start Printed Page 13573 Start Printed Page 13574 Start Printed Page 13575 Start Printed Page 13576 Start Printed Page 13577 Start Printed Page 13578 Start Printed Page 13579 Start Printed Page 13580 Start Printed Page 13581 Start Printed Page 13582 Start Printed Page 13583 Start Printed Page 13584 Start Printed Page 13585 Start Printed Page 13586 Start Printed Page 13587 Start Printed Page 13588 Start Printed Page 13589 Start Printed Page 13590 Start Printed Page 13591 Start Printed Page 13592 Start Printed Page 13593 Start Printed Page 13594 Start Printed Page 13595 Start Printed Page 13596 Start Printed Page 13597 Start Printed Page 13598 Start Printed Page 13599 Start Printed Page 13600 Start Printed Page 13601 Start Printed Page 13602 Start Printed Page 13603 Start Printed Page 13604 Start Printed Page 13605 Start Printed Page 13606 Start Printed Page 13607 Start Printed Page 13608 Start Printed Page 13609 Start Printed Page 13610 Start Printed Page 13611 Start Printed Page 13612 Start Printed Page 13613 Start Printed Page 13614 Start Printed Page 13615 Start Printed Page 13616 Start Printed Page 13617 Start Printed Page 13618 Start Printed Page 13619 Start Printed Page 13620 Start Printed Page 13621 Start Printed Page 13622 Start Printed Page 13623 Start Printed Page 13624 Start Printed Page 13625 Start Printed Page 13626 Start Printed Page 13627 Start Printed Page 13628 Start Printed Page 13629 Start Printed Page 13630 Start Printed Page 13631 Start Printed Page 13632 Start Printed Page 13633 Start Printed Page 13634 Start Printed Page 13635 Start Printed Page 13636 Start Printed Page 13637 Start Printed Page 13638 Start Printed Page 13639 Start Printed Page 13640 Start Printed Page 13641 Start Printed Page 13642 Start Printed Page 13643 Start Printed Page 13644 Start Printed Page 13645 Start Printed Page 13646 Start Printed Page 13647 Start Printed Page 13648 Start Printed Page 13649 Start Printed Page 13650 Start Printed Page 13651 Start Printed Page 13652 Start Printed Page 13653 Start Printed Page 13654 Start Printed Page 13655 Start Printed Page 13656 Start Printed Page 13657 Start Printed Page 13658 Start Printed Page 13659 Start Printed Page 13660 Start Printed Page 13661 Start Printed Page 13662 Start Printed Page 13663 Start Printed Page 13664 Start Printed Page 13665 Start Printed Page 13666 Start Printed Page 13667 Start Printed Page 13668 Start Printed Page 13669 Start Printed Page 13670 Start Printed Page 13671 Start Printed Page 13672 Start Printed Page 13673 Start Printed Page 13674 Start Printed Page 13675 Start Printed Page 13676 Start Printed Page 13677 Start Printed Page 13678 Start Printed Page 13679 Start Printed Page 13680 Start Printed Page 13681 Start Printed Page 13682 Start Printed Page 13683 Start Printed Page 13684 Start Printed Page 13685 Start Printed Page 13686 Start Printed Page 13687 Start Printed Page 13688 Start Printed Page 13689 Start Printed Page 13690 Start Printed Page 13691 Start Printed Page 13692 Start Printed Page 13693 Start Printed Page 13694 Start Printed Page 13695 Start Printed Page 13696 Start Printed Page 13697 Start Printed Page 13698 Start Printed Page 13699 Start Printed Page 13700 Start Printed Page 13701 Start Printed Page 13702 Start Printed Page 13703 Start Printed Page 13704 Start Printed Page 13705 Start Printed Page 13706 Start Printed Page 13707 Start Printed Page 13708 Start Printed Page 13709 Start Printed Page 13710 Start Printed Page 13711 Start Printed Page 13712 Start Printed Page 13713 Start Printed Page 13714 Start Printed Page 13715 Start Printed Page 13716 Start Printed Page 13717 Start Printed Page 13718 Start Printed Page 13719 Start Printed Page 13720 Start Printed Page 13721 Start Printed Page 13722 Start Printed Page 13723 Start Printed Page 13724 Start Printed Page 13725 Start Printed Page 13726 Start Printed Page 13727 Start Printed Page 13728 Start Printed Page 13729 Start Printed Page 13730 Start Printed Page 13731 Start Printed Page 13732 Start Printed Page 13733 Start Printed Page 13734 Start Printed Page 13735 Start Printed Page 13736 Start Printed Page 13737 Start Printed Page 13738 Start Printed Page 13739 Start Printed Page 13740 Start Printed Page 13741 Start Printed Page 13742 Start Printed Page 13743 Start Printed Page 13744 Start Printed Page 13745 Start Printed Page 13746 Start Printed Page 13747 Start Printed Page 13748 Start Printed Page 13749 Start Printed Page 13750 Start Printed Page 13751 Start Printed Page 13752 Start Printed Page 13753 Start Printed Page 13754 Start Printed Page 13755 Start Printed Page 13756 Start Printed Page 13757 Start Printed Page 13758 Start Printed Page 13759 Start Printed Page 13760 Start Printed Page 13761 Start Printed Page 13762 Start Printed Page 13763 Start Printed Page 13764 Start Printed Page 13765 Start Printed Page 13766 Start Printed Page 13767 Start Printed Page 13768 Start Printed Page 13769 Start Printed Page 13770 Start Printed Page 13771 Start Printed Page 13772 Start Printed Page 13773 Start Printed Page 13774 Start Printed Page 13775 Start Printed Page 13776 Start Printed Page 13777 Start Printed Page 13778 Start Printed Page 13779 Start Printed Page 13780 Start Printed Page 13781 Start Printed Page 13782 Start Printed Page 13783 Start Printed Page 13784 Start Printed Page 13785 Start Printed Page 13786 Start Printed Page 13787 Start Printed Page 13788 Start Printed Page 13789 Start Printed Page 13790 Start Printed Page 13791 Start Printed Page 13792 Start Printed Page 13793 Start Printed Page 13794 Start Printed Page 13795 Start Printed Page 13796 Start Printed Page 13797 Start Printed Page 13798 Start Printed Page 13799 Start Printed Page 13800 Start Printed Page 13801 Start Printed Page 13802 Start Printed Page 13803 Start Printed Page 13804 Start Printed Page 13805 Start Printed Page 13806 Start Printed Page 13807 Start Printed Page 13808 Start Printed Page 13809 Start Printed Page 13810 Start Printed Page 13811 Start Printed Page 13812 Start Printed Page 13813 Start Printed Page 13814 Start Printed Page 13815 Start Printed Page 13816 Start Printed Page 13817 Start Printed Page 13818 Start Printed Page 13819 Start Printed Page 13820 Start Printed Page 13821 Start Printed Page 13822 Start Printed Page 13823 Start Printed Page 13824 Start Printed Page 13825 Start Printed Page 13826 Start Printed Page 13827 Start Printed Page 13828 Start Printed Page 13829 Start Printed Page 13830 Start Printed Page 13831 Start Printed Page 13832 Start Printed Page 13833 Start Printed Page 13834 Start Printed Page 13835 Start Printed Page 13836 Start Printed Page 13837 Start Printed Page 13838 Start Printed Page 13839 Start Printed Page 13840 Start Printed Page 13841 Start Printed Page 13842 Start Printed Page 13843 Start Printed Page 13844 Start Printed Page 13845 Start Printed Page 13846 Start Printed Page 13847 Start Printed Page 13848 Start Printed Page 13849 Start Printed Page 13850 Start Printed Page 13851 Start Printed Page 13852 Start Printed Page 13853 Start Printed Page 13854 Start Printed Page 13855 Start Printed Page 13856 Start Printed Page 13857 Start Printed Page 13858 Start Printed Page 13859 Start Printed Page 13860 Start Printed Page 13861 Start Printed Page 13862 Start Printed Page 13863 Start Printed Page 13864 Start Printed Page 13865 Start Printed Page 13866 Start Printed Page 13867 Start Printed Page 13868 Start Printed Page 13869 Start Printed Page 13870 Start Printed Page 13871 Start Printed Page 13872 Start Printed Page 13873 Start Printed Page 13874 Start Printed Page 13875 Start Printed Page 13876 Start Printed Page 13877 Start Printed Page 13878 Start Printed Page 13879 Start Printed Page 13880 Start Printed Page 13881 Start Printed Page 13882 Start Printed Page 13883 Start Printed Page 13884 Start Printed Page 13885 Start Printed Page 13886 Start Printed Page 13887 Start Printed Page 13888 Start Printed Page 13889 Start Printed Page 13890 Start Printed Page 13891 Start Printed Page 13892 Start Printed Page 13893 Start Printed Page 13894 Start Printed Page 13895 Start Printed Page 13896 Start Printed Page 13897 Start Printed Page 13898 Start Printed Page 13899 Start Printed Page 13900 Start Printed Page 13901 Start Printed Page 13902 Start Printed Page 13903 Start Printed Page 13904 Start Printed Page 13905 Start Printed Page 13906 Start Printed Page 13907 Start Printed Page 13908 Start Printed Page 13909 Start Printed Page 13910 Start Printed Page 13911 Start Printed Page 13912 Start Printed Page 13913 Start Printed Page 13914 Start Printed Page 13915 Start Printed Page 13916 Start Printed Page 13917 Start Printed Page 13918 Start Printed Page 13919 Start Printed Page 13920 Start Printed Page 13921 Start Printed Page 13922 Start Printed Page 13923 Start Printed Page 13924 End Supplemental InformationDated: March 16, 2009.
Jacquelyn Y. White,
Director, Office of Strategic Operations and Regulatory Affairs.
[FR Doc. E9-6148 Filed 3-26-09; 8:45 am]
BILLING CODE 4120-01-P
Document Information
- Published:
- 03/27/2009
- Department:
- Centers for Medicare & Medicaid Services
- Entry Type:
- Notice
- Action:
- Notice.
- Document Number:
- E9-6148
- Pages:
- 13515-13924 (410 pages)
- Docket Numbers:
- CMS-9050-N
- PDF File:
- e9-6148.pdf
- Supporting Documents:
- » Single Source Funding Opportunity: Comprehensive Patient Reported Survey for Mental and Behavioral Health
- » Performance Review Board Membership
- » Single Source Award: Analyses, Research, and Studies to Assess the Impact of Centers for Medicare and Medicaid Services Programs on American Indians/Alaska Natives and the Indian Health Care System Serving American Indians/Alaska Natives Beneficiaries
- » Privacy Act; Matching Program
- » Nondiscrimination in Health Programs and Activities
- » Survey, Certification, and Enforcement Procedures; CFR Correction
- » Securing Updated and Necessary Statutory Evaluations Timely; Withdrawal
- » Securing Updated and Necessary Statutory Evaluations Timely; Administrative Delay of Effective Date
- » Medicare Program: Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals; Changes to Medicare Graduate Medical Education Payments for Teaching Hospitals; Changes to Organ Acquisition Payment Policies
- » Medicare Program; Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Policy Issues, and Level II of the Healthcare Common Procedure Coding System (HCPCS); DME Interim Pricing in the CARES Act; Durable Medical Equipment Fee Schedule Adjustments To Resume the Transitional 50/50 Blended Rates To Provide Relief in Rural Areasand Non-Contiguous Areas